Abstract
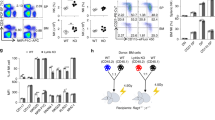
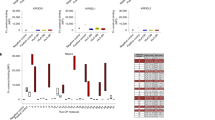
The development and function of natural killer (NK) cells is regulated by the interaction of inhibitory receptors of the Ly49 family with distinct peptide-laden major histocompatibility complex (MHC) class I molecules, although whether the Ly49 family is able bind to other MHC class I–like molecules is unclear. Here we found that the prototypic inhibitory receptor Ly49A bound the highly conserved nonclassical MHC class I molecule H2-M3 with an affinity similar to its affinity for H-2Dd. The specific recognition of H2-M3 by Ly49A regulated the 'licensing' of NK cells and mediated 'missing-self' recognition of H2-M3-deficient bone marrow. Host peptide–H2-M3 was required for optimal NK cell activity against experimental metastases and carcinogenesis. Thus, nonclassical MHC class I molecules can act as cognate ligands for Ly49 molecules. Our results provide insight into the various mechanisms that lead to NK cell tolerance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
19 November 2012
In the version of this article initially published, two designations for major histocompatibility complex molecules in the second subsection of Results are incorrect. The correct text should read "The affinity of AGPARAAAL-loaded H-2Dd for Ly49A (2.05 ± 0.07 μM; Fig. 2c) was similar to published values36. In contrast, we observed no interaction between Ly49A and SIINFEKL-loaded H-2Kb (Fig. 2d)...." The error has been corrected for HTML and PDF versions of this article.
References
Chan, C.J. et al. Can NK cells be a therapeutic target in human cancer? Eur. J. Immunol. 38, 2964–2968 (2008).
Ljunggren, H.G. & Malmberg, K.J. Prospects for the use of NK cells in immunotherapy of human cancer. Nat. Rev. Immunol. 7, 329–339 (2007).
Andoniou, C.E. et al. Natural killer cells in viral infection: more than just killers. Immunol. Rev. 214, 239–250 (2006).
Karlhofer, F.M. et al. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature 358, 66–70 (1992).
Yu, Y.Y. et al. The role of Ly49A and 5E6(Ly49C) molecules in hybrid resistance mediated by murine natural killer cells against normal T cell blasts. Immunity 4, 67–76 (1996).
Kärre, K. et al. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 319, 675–678 (1986).
Kärre, K. How to recognize a foreign submarine. Immunol. Rev. 155, 5–9 (1997).
Natarajan, K. et al. Interaction of the NK cell inhibitory receptor Ly49A with H-2Dd: identification of a site distinct from the TCR site. Immunity 11, 591–601 (1999).
Held, W. et al. Major histocompatibility complex class I-dependent skewing of the natural killer cell Ly49 receptor repertoire. Eur. J. Immunol. 26, 2286–2292 (1996).
Valiante, N.M. et al. Functionally and structurally distinct NK cell receptor repertoires in the peripheral blood of two human donors. Immunity 7, 739–751 (1997).
Hanke, T. et al. Direct assessment of MHC class I binding by seven Ly49 inhibitory NK cell receptors. Immunity 11, 67–77 (1999).
Fernandez, N.C. et al. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood 105, 4416–4423 (2005).
Höglund, P. et al. Recognition of β2-microglobulin-negative (β2m−) T-cell blasts by natural killer cells from normal but not from β2m− mice: nonresponsiveness controlled by β2m− bone marrow in chimeric mice. Proc. Natl. Acad. Sci. USA 88, 10332–10336 (1991).
Raulet, D.H. & Vance, R.E. Self-tolerance of natural killer cells. Nat. Rev. Immunol. 6, 520–531 (2006).
Kim, S. et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 436, 709–713 (2005).
Kumar, V. & McNerney, M.E. A new self: MHC-class-I-independent natural-killer-cell self-tolerance. Nat. Rev. Immunol. 5, 363–374 (2005).
Vance, R.E. et al. Mouse CD94/NKG2A is a natural killer cell receptor for the nonclassical major histocompatibility complex (MHC) class I molecule Qa-1b. J. Exp. Med. 188, 1841–1848 (1998).
Hanke, T. et al. Identification of an immunodominant cytotoxic T-lymphocyte recognition site in glycoprotein B of herpes simplex virus by using recombinant adenovirus vectors and synthetic peptides. J. Virol. 65, 1177–1186 (1991).
Borrego, F. et al. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence-derived peptides by CD94/NKG2 confers protection from natural killer cell-mediated lysis. J. Exp. Med. 187, 813–818 (1998).
Braud, V.M. et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 391, 795–799 (1998).
Ohtsuka, M. et al. Major histocompatibility complex (MHC) class Ib gene duplications, organization and expression patterns in mouse strain C57BL/6. BMC Genomics 9, 178 (2008).
Chiu, N.M. et al. The majority of H2–M3 is retained intracellularly in a peptide-receptive state and traffics to the cell surface in the presence of N-formylated peptides. J. Exp. Med. 190, 423–434 (1999).
Loveland, B. et al. Maternally transmitted histocompatibility antigen of mice: a hydrophobic peptide of a mitochondrially encoded protein. Cell 60, 971–980 (1990).
Gulden, P.H. et al. A Listeria monocytogenes pentapeptide is presented to cytolytic T lymphocytes by the H2–M3 MHC class Ib molecule. Immunity 5, 73–79 (1996).
Lenz, L.L. et al. Identification of an H2–M3-restricted Listeria epitope: implications for antigen presentation by M3. Immunity 5, 63–72 (1996).
Wang, C.R. et al. H-2M3 encodes the MHC class I molecule presenting the maternally transmitted antigen of the mouse. Cell 66, 335–345 (1991).
Colmone, A. & Wang, C.-R. H2–M3-restricted T cell response to infection. Microbes Infect. 8, 2277–2283 (2006).
Xu, H. et al. Impaired response to Listeria in H2–M3-deficient mice reveals a nonredundant role of MHC class Ib-specific T cells in host defense. J. Exp. Med. 203, 449–459 (2006).
Deng, L. et al. Molecular architecture of the major histocompatibility complex class I-binding site of Ly49 natural killer cell receptors. J. Biol. Chem. 283, 16840–16849 (2008).
Brennan, J. et al. Recognition of class I major histocompatibility complex molecules by Ly-49: specificities and domain interactions. J. Exp. Med. 183, 1553–1559 (1996).
Brodin, P. et al. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood 113, 2434–2441 (2009).
Johansson, S. et al. Probing natural killer cell education by Ly49 receptor expression analysis and computational modelling in single MHC class I mice. PLoS ONE 4, e6046 (2009).
Michaëlsson, J. et al. Visualization of inhibitory Ly49 receptor specificity with soluble major histocompatibility complex class I tetramers. Eur. J. Immunol. 30, 300–307 (2000).
Scarpellino, L. et al. Interactions of Ly49 family receptors with MHC class I ligands in trans and cis. J. Immunol. 178, 1277–1284 (2007).
Burrows, S.R. et al. Peptide-MHC class I tetrameric complexes display exquisite ligand specificity. J. Immunol. 165, 6229–6234 (2000).
Wang, J. et al. Binding of the natural killer cell inhibitory receptor Ly49A to its major histocompatibility complex class I ligand. Crucial contacts include both H-2Dd AND β2-microglobulin. J. Biol. Chem. 277, 1433–1442 (2002).
Zafirova, B. et al. Altered NK cell development and enhanced NK cell-mediated resistance to mouse cytomegalovirus in NKG2D-deficient mice. Immunity 31, 270–282 (2009).
Hayakawa, Y. & Smyth, M.J. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J. Immunol. 176, 1517–1524 (2006).
Guerra, N. et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 28, 571–580 (2008).
Joncker, N.T. et al. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J. Exp. Med. 207, 2065–2072 (2010).
Sun, K. et al. Mouse NK cell-mediated rejection of bone marrow allografts exhibits patterns consistent with Ly49 subset licensing. Blood 119, 1590–1598 (2012).
Oberg, L. et al. Loss or mismatch of MHC class I is sufficient to trigger NK cell-mediated rejection of resting lymphocytes in vivo: role of KARAP/DAP12-dependent and -independent pathways. Eur. J. Immunol. 34, 1646–1653 (2004).
Chan, P.Y. & Takei, F. Expression of a T cell receptor-like molecule on normal and malignant murine T cells detected by rat monoclonal antibodies to nonclonotypic determinants. J. Immunol. 136, 1346–1353 (1986).
Nagasawa, R. et al. Identification of a novel T cell surface disulfide-bonded dimer distinct from the α/β antigen receptor. J. Immunol. 138, 815–824 (1987).
Wong, S. et al. Ly-49 multigene family. New members of a superfamily of type II membrane proteins with lectin-like domains. J. Immunol. 147, 1417–1423 (1991).
Jonsson, A.H. et al. Effects of MHC class I alleles on licensing of Ly49A+ NK cells. J. Immunol. 184, 3424–3432 (2010).
Brodin, P. et al. Skewing of the NK cell repertoire by MHC class I via quantitatively controlled enrichment and contraction of specific Ly49 subsets. J. Immunol. 188, 2218–2226 (2012).
Elliott, J.M. & Yokoyama, W.M. Unifying concepts of MHC-dependent natural killer cell education. Trends Immunol. 32, 364–372 (2011).
Hoare, H.L. et al. Structural basis for a major histocompatibility complex class Ib-restricted T cell response. Nat. Immunol. 7, 256–264 (2006).
Pellicci, D.G. et al. Differential recognition of CD1d-α-galactosyl ceramide by the Vβ8.2 and Vβ7 semi-invariant NKT T cell receptors. Immunity 31, 47–59 (2009).
Acknowledgements
We thank C. Paget for critical analysis of the manuscript; E. Hawkins, Q. Mundrea, B. Venville, N. McLaughlin and J. Sharkey for technical assistance; and B. Murphy (UC Davis Cancer Center) for the YE1/32 antibody to Ly49A. Supported by the National Health and Medical Research Council of Australia (D.M.A., L.C.S., R.B., M.J.S., A.G.B. and J.R.), Cancer Australia, the Cure Cancer Australia Foundation, the US National Institutes of Health (AI040310 to C.-R.W.) and the Howard Hughes Medical Institute (W.M.Y.).
Author information
Authors and Affiliations
Contributions
D.M.A. conceived of, designed and did the experiments with assistance from L.C.S., N.B., C.J.C., R.B., C.L.C., H.H., J.L., S.V.W., J.P.-L. and M.J.S.; C.-R.W., A.A.S., W.M.Y., J.R., A.G.B. and M.J.S. provided reagents and critical input to the analysis of results; and D.M.A., L.C.S., J.R., A.G.B. and M.J.S. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 (PDF 165 kb)
Rights and permissions
About this article
Cite this article
Andrews, D., Sullivan, L., Baschuk, N. et al. Recognition of the nonclassical MHC class I molecule H2-M3 by the receptor Ly49A regulates the licensing and activation of NK cells. Nat Immunol 13, 1171–1177 (2012). https://doi.org/10.1038/ni.2468
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2468
This article is cited by
-
Synergized regulation of NK cell education by NKG2A and specific Ly49 family members
Nature Communications (2019)
-
Recognition of host Clr-b by the inhibitory NKR-P1B receptor provides a basis for missing-self recognition
Nature Communications (2018)
-
NK cell education via nonclassical MHC and non-MHC ligands
Cellular & Molecular Immunology (2017)
-
Characterisation of major histocompatibility complex class I genes at the fetal-maternal interface of marsupials
Immunogenetics (2015)
-
The Ly49 natural killer cell receptors: a versatile tool for viral self‐discrimination
Immunology & Cell Biology (2014)