Abstract
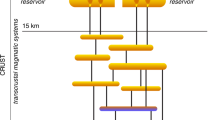
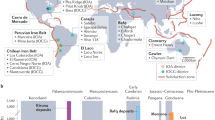
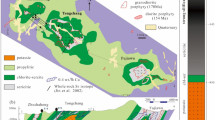
Porphyry copper deposits, that is, copper ore associated with hydrothermal fluids rising from a magma chamber, supply 75% of the world’s copper. They are typically associated with intrusions of magma in the crust above subduction zones, indicating a primary role for magmatism in driving mineralization. However, it is not clear that a single, copper-rich magmatic fluid could trigger both copper enrichment and the subsequent precipitation of sulphide ore minerals within a zone of hydrothermally altered rock. Here we draw on observations of modern subduction zone volcanism to propose an alternative process for porphyry copper formation. We suggest that copper enrichment initially involves metalliferous, magmatic hyper-saline liquids, or brines, that exsolve from large, magmatic intrusions assembled in the shallow crust over tens to hundreds of thousands of years. In a subsequent step, sulphide ore precipitation is triggered by the interaction of the accumulated brines with sulphur-rich gases, liberated in short-lived bursts from the underlying mafic magmas. We use high-temperature and high-pressure laboratory experiments to simulate such gas–brine interactions. The experiments yield copper–iron sulphide minerals and hydrogen chloride gas at magmatic temperatures of 700–800 °C, with textural and chemical characteristics that resemble those in porphyry copper deposits. We therefore conclude that porphyry copper ore forms in a two-stage process of brine enrichment followed by gas-induced precipitation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hedenquist, J. W. & Lowenstern, J. B. The role of magmas in the formation of hydrothermal ore deposits. Nature 370, 519–527 (1994).
Sillitoe, R. H. Porphyry copper systems. Econ. Geol. 105, 3–41 (2010).
Cline, J. S. & Bodnar, R. J. Can economic porphyry copper mineralization be generated by a typical calc-alkaline melt? J. Geophys. Res. 96, 8113–8126 (1991).
Landtwing, M. R. et al. Copper deposition during quartz dissolution by cooling magmatic–hydrothermal fluids: The Bingham porphyry. Earth Planet. Sci. Lett. 235, 229–243 (2005).
Weis, P., Driesner, T. & Heinrich, C. A. Porphyry-copper ore shells form at stable pressure–temperature fronts within dynamic fluid plumes. Science 338, 1613–1616 (2012).
Halter, W. E., Heinrich, C. A. & Pettke, T. Magma evolution and the formation of porphyry Cu–Au ore fluids: Evidence from silicate and sulfide melt inclusions. Mineral. Deposita 39, 845–863 (2005).
Wilkinson, J. J. Triggers for the formation of porphyry ore deposits in magmatic arcs. Nature Geosci. 6, 917–925 (2013).
Richards, J. P. Giant ore deposits formed by optimal alignment and combination of geological processes. Nature Geosci. 6, 911–916 (2013).
Crerar, D. A. & Barnes, H. L. Ore solution chemistry V. Solubilities of chalcopyrite and chalcocite assemblages in hydrothermal solution at 200 to 350 °C. Econ. Geol. 71, 772–794 (1976).
Hemley, J. J., Cygan, G. L., Fein, J. B., Robinson, G. R. & D’Angelo, W. M. Hydrothermal ore-forming processes in the light of studies in rock-buffered systems I:. Iron–copper–zinc–lead sulfide solubility relations. Econ. Geol. 87, 1–22 (1992).
Lee, C. A. et al. Copper systematics in arc magmas and implications for crust–mantle differentiation. Science 336, 64–68 (2012).
Edmonds, M. New geochemical insights into volcanic degassing. Phil. Trans. R. Soc. A 366, 4559–4579 (2008).
Shinohara, H. Exsolution of immiscible vapor and liquid phases from a crystallizing silicate melt: Implications for chlorine and metal transport. Geochim. Cosmochim. Acta 58, 5215–5221 (1994).
Hattori, K. H. & Keith, J. D. Contribution of mafic melt to porphyry copper mineralization: Evidence from Mount Pinatubo, Philippines, and Bingham Canyon, Utah, USA. Mineral. Deposita 36, 799–806 (2001).
Wallace, P. J. Volatiles in subduction zone magmas: Concentrations and fluxes based on melt inclusion and volcanic gas data. J. Volcanol. Geotherm. Res. 140, 217–240 (2005).
Christopher, T., Edmonds, M., Humphreys, M. C. S. & Herd, R. A. Volcanic gas emissions from Soufrière Hills Volcano, Montserrat 1995–2009, with implications for mafic magma supply and degassing. Geophys. Res. Lett. 37, LE00E04 (2010).
Lesne, P. et al. Experimental simulation of closed-system degassing in the system basalt–H2O–CO2–S–Cl. J. Petrol. 52, 1737–1762 (2011).
Keppler, H. The distribution of sulfur between haplogranitic melts and aqueous fluids. Geochim. Cosmochim. Acta 74, 645–660 (2010).
Pyle, D. M. & Mather, T. A. Halogens in igneous processes and their fluxes to the atmosphere and oceans from volcanic activity: A review. Chem. Geol. 263, 110–121 (2009).
Bodnar, R. J., Lecumberri-Sanchez, P., Moncada, D. & Steele-MacInnis, M. Treatise on Geochemistry 2nd edn, Ch. 13.5, 119–142 (Elsevier, 2014).
Simon, A. C., Pettke, T., Candela, P. A., Piccoli, P. M. & Heinrich, C. A. Copper partitioning in a melt–vapor–brine–magnetite–pyrrhotite assemblage. Geochim. Cosmochim. Acta 70, 5583–5600 (2006).
Tattitch, B. C., Candela, P. A., Piccoli, P. M. & Bodnar, R. J. Copper partitioning between felsic melt and H2O–CO2 bearing saline fluids. Geochim. Cosmochim. Acta 148, 81–99 (2014).
Borisova, A. Y. et al. Trace element geochemistry of the 1991 Mt. Pinatubo silicic melts, Philippines: Implications for ore-forming potential of adakitic magmatism. Geochim. Cosmochim. Acta 70, 3702–3716 (2006).
Blundy, J., Cashman, K. & Berlo, K. Evolving magma storage conditions beneath Mount St. Helens inferred from chemical variations in melt inclusions from the 1980–1986 and current eruptions. USGS Prof. Pap. 1750, 755–790 (2008).
Kasai, K., Sakagawa, Y., Miyazaki, S., Akaku, K. & Uchida, T. Supersaline and metal-rich brine obtained from the Quaternary Kakkonda Granite by NEDO WD-1a in the Kakkonda geothermal field, Japan. Mineral. Deposita 33, 298–301 (1998).
Valori, A., Cathelineau, M. & Marignac, C. Early fluid migration in a deep part of the Larderello geothermal field: A fluid inclusion study of the granite sill from well Monteverdi 7. J. Volcanol. Geotherm. Res. 51, 115–131 (1992).
Paulatto, M. et al. Upper crustal structure of an active volcano from refraction/reflection tomography, Montserrat, Lesser Antilles. Geophys. J. Int. 180, 685–696 (2009).
Kagiyama, T., Utada, H. & Yamamoto, T. Magma ascent beneath Unzen Volcano, SW Japan, deduced from the electrical resistivity structure. J. Volcanol. Geotherm. Res. 89, 35–42 (1999).
Müller, A. & Haak, V. 3-D modeling of the deep electrical conductivity of Merapi volcano (Central Java): Integrating magnetotellurics, induction vectors and the effects of steep topography. J. Volcanol. Geotherm. Res. 138, 205–222 (2004).
Seo, J. H., Guillong, M. & Heinrich, C. A. The role of sulfur in the formation of magmatic–hydrothermal copper–gold deposits. Earth Planet. Sci. Lett. 282, 323–328 (2009).
Seo, J. H. & Heinrich, C. A. Selective copper diffusion into quartz-hosted vapor inclusions: Evidence from other host minerals, driving forces, and consequences for Cu–Au ore formation. Geochim. Cosmochim. Acta 113, 60–69 (2013).
Burgisser, A. & Scaillet, B. Redox evolution of a degassing magma rising to the surface. Nature 445, 194–197 (2007).
Newton, R. C. & Manning, C. E. Solubility of anhydrite, CaSO4, in NaCl–H2O solutions at high pressures and temperatures: Applications to fluid–rock interaction. J. Petrol. 46, 701–716 (2005).
Blundy, J., Cashman, K., Rust, A. & Witham, F. A case for CO2-rich arc magmas. Earth Planet. Sci. Lett. 290, 289–301 (2010).
Mei, Y., Sherman, D. M., Liu, W. & Brugger, J. Ab initio molecular dynamics simulation and free energy exploration of copper(I) complexation by chloride and bisulfide in hydrothermal fluids. Geochim. Cosmochim. Acta 102, 45–64 (2013).
Giggenbach, W. F. in Geochemistry of Hydrothermal Ore Deposits 3rd edn (ed. Barnes, H. L.) Ch. 15, 737–796 (Wiley, 1997).
Seward, T. M. & Barnes, H. L. in Geochemistry of Hydrothermal Ore Deposits 3rd edn (ed. Barnes, H. L.) Ch. 9, 435–486 (Wiley, 1997).
Frank, M. R., Candela, P. A. & Piccoli, P. M. K–feldspar–muscovite–andalusite–quartz–brine phase equilibria: An experimental study at 25 to 60 MPa and 400 to 550 °C. Geochim. Cosmochim. Acta 62, 3717–3727 (1998).
John, D. A. (ed.) Porphyry Copper Deposit Model: Chapter B of Mineral Deposit Models for Resource Assessment Report 20105070B (USGS, 2010).
Gustafson, L. B. Some major factors of porphyry copper genesis. Econ. Geol. 73, 600–607 (1978).
Caricchi, L., Simpson, G. & Schaltegger, U. Zircons reveal magma fluxes in the Earth’s crust. Nature 511, 457–461 (2014).
Frank, M. R. & Vaccaro, D. M. An experimental study of high temperature potassic alteration. Geochim. Cosmochim. Acta 83, 195–204 (2012).
Crisp, J. Rates of magma emplacement and volcanic output. J. Volcanol. Geotherm. Res. 20, 177–211 (1984).
Luhr, J. F., Carmichael, I. S. E. & Varekamp, J. C. The 1982 eruptions of El Chichón Volcano, Chiapas, Mexico: Mineralogy and petrology of the anhydrite-bearing pumices. J. Volcanol. Geotherm. Res. 23, 69–108 (1984).
Plail, M., Edmonds, M., Humphreys, M. C. S., Barclay, J. & Herd, R. A. Geochemical evidence for relict degassing pathways preserved in andesite. Earth Planet. Sci. Lett. 386, 21–33 (2014).
McCormick, B. T. et al. Volcano monitoring applications of the Ozone Monitoring Instrument. Geol. Soc. Lond. 380, 259–291 (2013).
Blundy, J. D. & Sparks, R. S. J. Petrogenesis of mafic inclusions in granitoids of the Adamello Massif, Italy. J. Petrol. 33, 1039–1104 (1992).
Acknowledgements
We acknowledge research funding from BHP Billiton, a Benjamin Meaker Visiting Professorship to J.M. and a Royal Society Wolfson Research Merit Award and ERC Advanced Grant (CRITMAG) to J.B. This work has benefited from discussions with J. Dilles, D. Dolejs, J. Eiler, C. Ford, R. Gold, R. Henley, C. Heinrich, J. Price, D. Sherman, E. Stolper, A. Webb and G. Yeates, as well as members of the Caltech PRG. We are grateful to M. Pistone for synthesizing the starting materials and R. Brooker and B. Buse for technical assistance.
Author information
Authors and Affiliations
Contributions
J.B. developed the hypothesis in conjunction with B.T. J.M. performed the experiments and analysed the run products. B.T. characterized the synthetic fluid inclusions. A.G. made observations of the Don Manuel core. J.B. wrote the first draft of the manuscript; all authors assisted in producing the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 443 kb)
Rights and permissions
About this article
Cite this article
Blundy, J., Mavrogenes, J., Tattitch, B. et al. Generation of porphyry copper deposits by gas–brine reaction in volcanic arcs. Nature Geosci 8, 235–240 (2015). https://doi.org/10.1038/ngeo2351
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo2351
This article is cited by
-
Magma mixing and magmatic-to-hydrothermal fluid evolution revealed by chemical and boron isotopic signatures in tourmaline from the Zhunuo–Beimulang porphyry Cu-Mo deposits
Mineralium Deposita (2024)
-
Formation of giant iron oxide-copper-gold deposits by superimposed episodic hydrothermal pulses
Scientific Reports (2023)
-
Water-sulfur-rich, oxidised adakite magmas are likely porphyry copper progenitors
Scientific Reports (2023)
-
Microthermometry and noble gas isotope analysis of magmatic fluid inclusions in the Kerman porphyry Cu deposits, Iran: constraints on the source of ore-forming fluids
Mineralium Deposita (2022)
-
Combined Feldspar-Destructive Processes and Hypogene Sulfide Mineralization in the Porphyry Copper Systems: Potentials for Geochemical Signals of Ore Discovering
Iranian Journal of Science and Technology, Transactions A: Science (2022)