Abstract
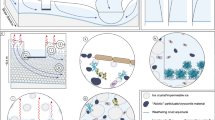
Glaciers and ice sheets are melting in response to climate warming. Whereas the physical behaviour of glaciers has been studied intensively, the biological processes associated with glaciers and ice sheets have received less attention. Nevertheless, field observations and laboratory experiments suggest that biological processes that occur on the surface of glaciers and ice sheets — collectively termed supraglacial environments — can affect the physical behaviour of glaciers by changing surface reflectivity. Furthermore, supraglacial cyanobacteria and algae capture carbon dioxide from the atmosphere and convert it into organic matter. Supraglacial microbes break down this material, together with organic matter transported from further afield, and generate carbon dioxide that is released back into the atmosphere. The balance between these two processes will determine whether a glacier is a net sink or source of carbon dioxide. In general, ice sheet interiors seem to function as sinks, whereas ice sheet edges and small glaciers act as a source. Meltwaters flush microbially modified organic matter and pollutants out of the glacier, with potential consequences for downstream ecosystems. We conclude that microbes living on glaciers and ice sheets are an integral part of both the glacial environment and the Earth's ecosystem.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Benn, D. I. & Evans, D. J. A. Glaciers and glaciation (Arnold, London, 1998).
Hanna, E. et al. Runoff and mass balance of the Greenland ice sheet: 1958–2003 J. Geophys. Res. 110, D13108 (2005).
Anesio, A. M., Hodson, A. J., Fritz, A., Psenner, R. & Sattler, B. High microbial activity on glaciers: importance to the global carbon cycle. Glob. Change Biol. 15, 955–960 (2009).
Hodson, A. et al. The cryoconite ecosystem on the Greenland ice sheet. Ann. Glaciol. 51, 123–129 (2010).
Lenaerts, J. T. M., van den Broeke, M. R., van de Berg, W. J., van Meijgaard, E. & Kuipers Munneke, P. A new, high-resolution surface mass balance map of Antarctica (1979–2010) based on regional atmospheric climate modeling. Geophys. Res. Lett. 39, L04501 (2012).
Price, P. B., Rohde, R. A. & Bay, R. C. Fluxes of microbes, organic aerosols, dust, sea-salt Na ions, non-sea-salt Ca ions, and methanesulfonate onto Greenland and Antarctic ice. Biogeosciences 6, 479–486 (2009).
Remias, D., Holzinger, A. & Lütz, C. Physiology, ultrastructure and habitat of the ice alga Mesotaenium berggrenii (Zygnemaphyceae, Chlorophyta) from glaciers in the European Alps. Phycologia 48, 302–312 (2009).
Langford, H., Hodson, A., Banwart, S. & Bøggild, C. The microstructure and biogeochemistry of Arctic cryoconite granules. Ann. Glaciol. 51, 87–94 (2010).
Takeuchi, N. Temporal and spatial variations in spectral reflectance and characteristics of surface dust on Gulkana Glacier, Alaska Range. J. Glaciol. 55, 701–709 (2009).
Hodson, A. et al. The structure, biological activity and biogeochemistry of cryoconite aggregates upon an Arctic valley glacier: Longyearbreen, Svalbard. J. Glaciol. 56, 349–361 (2010).
Stibal, M., Šabacká, M. & Kaštovská, K. Microbial communities on glacier surfaces in Svalbard: Impact of physical and chemical properties on abundance and structure of cyanobacteria and algae. Microb. Ecol. 52, 644–654 (2006).
Bøggild, C. E., Brandt, R. E., Brown, K. J. & Warren, S. G. The ablation zone in northeast Greenland: ice types, albedos and impurities. J. Glaciol. 56, 101–113 (2010).
Wientjes, I. G. M., van de Wal, R. S. W., Reichart, G. J., Sluijs, A. & Oerlemans, J. Dust from the dark region in the western ablation zone of the Greenland ice sheet. Cryosphere 5, 589–601 (2011).
Box, J. E. et al. Greenland ice sheet albedo feedback: thermodynamics and atmospheric drivers. Cryosphere 6, 821–839 (2012).
Takeuchi, N. & Li, Z. Characteristics of surface dust on Ürümqi Glacier No. 1 in the Tien Shan Mountains, China. Arct. Antarct. Alp. Res. 40, 744–750 (2008).
Yallop, M. L. et al. Photophysiology and albedo-changing potential of the ice algae community on the surface of Greenland Ice Sheet. ISME J. http://dx.doi.org/10.1038/ismej.2012.107 (2012).
Remias, D. et al. Characterization of an UV- and VIS-absorbing, purpurogallin-derived secondary pigment new to algae and highly abundant in Mesotaenium berggrenii (Zygnematophyceae, Chlorophyta), an extremophyte living on glaciers. FEMS Microbiol. Ecol. 79, 638–648 (2012).
Foreman, C. M., Sattler, B., Mikucki, J. A., Porazinska, D. L. & Priscu, J. C. Metabolic activity and diversity of cryoconites in the Taylor Valley, Antarctica. J. Geophys. Res. 112, G04S32 (2007).
Hodson A. et al. A glacier respires: quantifying the distribution and respiration CO2 flux of cryoconite across an entire Arctic supraglacial ecosystem. J. Geophys. Res. 112, G04S36 (2007).
Stibal, M., Tranter, M., Benning, L. G. & Řehák, J. Microbial primary production on an Arctic glacier is insignificant in comparison with allochthonous organic carbon input. Environ. Microbiol. 10, 2172–2178 (2008).
Anesio, A. M. et al. Carbon fluxes through bacterial communities on glacier surfaces. Ann. Glaciol. 51, 32–40 (2010).
Stibal, M. et al. Environmental controls on microbial abundance and activity on the Greenland ice sheet: a multivariate analysis approach. Microb. Ecol. 63, 74–84 (2012).
Edwards, A. et al. Possible interactions between bacterial diversity, microbial activity and supraglacial hydrology of cryoconite holes in Svalbard. ISME J. 5, 150–160 (2011).
Bagshaw, E. A., Tranter, M., Wadham, J. L., Fountain, A. G. & Mowlem, M. High-resolution monitoring reveals dissolved oxygen dynamics in an Antarctic cryoconite hole. Hydrol. Process. 25, 2868–2877 (2011).
Säwström, C., Laybourn-Parry, J., Granéli, W. & Anesio, A. M. Heterotrophic bacterial and viral dynamics in Arctic freshwaters: results from a field study and nutrient-temperature manipulation experiments. Polar Biol. 30, 1407–1415 (2007).
Telling, J. et al. Microbial nitrogen cycling on the Greenland Ice Sheet. Biogeosciences 9, 2431–2442 (2012).
Segawa, T. & Takeuchi, N. Cyanobacterial communities on Qiyi glacier, Qilian Shan, China. Ann. Glaciol. 51, 135–144 (2010).
Uetake, J., Naganuma, T., Hebsgaard, M. B., Kanda, H. & Kohshima, S. Communities of algae and cyanobacteria on glaciers in west Greenland. Polar Sci. 4, 71–80 (2010).
Telling, J. et al. Controls on the autochthonous production and respiration of organic matter in cryoconite holes on High Arctic glaciers. J. Geophys. Res. 117, G01017 (2012).
Tranter, M. et al. Extreme hydrochemical conditions in natural microcosms entombed within Antarctic ice. Hydrol. Process. 18, 379–387 (2004).
Cook, J. et al. The mass–area relationship within cryoconite holes and its implications for primary production. Ann. Glaciol. 51, 106–110 (2010).
Takeuchi, N., Nishiyama, H. & Li, Z. Structure and formation process of cryoconite granules on Ürümqi glacier No. 1, Tien Shan, China. Ann. Glaciol. 51, 9–14 (2010).
Xiang, S.-R., Shang T.-C., Chen, Y., Jing, Z.-F. & Yao, T. Dominant bacteria and biomass in the Kuytun 51 Glacier. Appl. Environ. Microbiol. 75, 7287–7290 (2009).
Cameron, K. A., Hodson, A. J. & Osborn, A. M. Structure and diversity of bacterial, eukaryotic and archaeal communities in glacial cryoconite holes from the Arctic and the Antarctic. FEMS Microbiol. Ecol. http://dx.doi.org/10.1111/j.1574-6941.2011.01277.x (2011).
Bhatia, M. P., Das, S. B., Longnecker, K., Charette, M. A. & Kujawinski, E. B. Molecular characterization of dissolved organic matter associated with the Greenland ice sheet. Geochim. Cosmochim. Acta 74, 3768–3784 (2010).
Xu, Y., Simpson, A. J., Eyles, N. & Simpson, M. J. Sources and molecular composition of cryoconite organic matter from the Athabasca Glacier, Canadian Rocky Mountains. Org. Geochem. 41, 177–186 (2010).
Grannas, A. M., Hockaday, W. C., Hatcher, P. G., Thompson, L. G. & Mosley-Thompson, E. New revelations on the nature of organic matter in ice cores. J. Geophys. Res. 111, D04304 (2006).
Margesin, R., Zacke, G. & Schinner, F. Characterization of heterotrophic microorganisms in alpine glacier cryoconite. Arct. Antarct. Alp. Res. 34, 88–93 (2002).
Bogdal, C. et al. Blast from the past: Melting glaciers as a relevant source for persistent organic pollutants. Environ. Sci. Technol. 43, 8173–8177 (2009).
Stibal, M. et al. Microbial degradation of 2,4-dichlorophenoxyacetic acid on the Greenland ice sheet. Appl. Environ. Microbiol. 78, 5070–5076 (2012).
Telling, J. et al. Measuring rates of gross photosynthesis and net community production in cryoconite holes: a comparison of field methods. Ann. Glaciol. 51 (56), 135–144 (2010).
Antony, R., Mahalinganathan, K., Thamban, M. & Nair S. Organic carbon in Antarctic snow: spatial trends and possible sources. Environ. Sci. Technol. 45, 9944–9950 (2011).
Stubbins, A. et al. Anthropogenic aerosols as a source of ancient dissolved organic matter in glaciers. Nat. Geosci. 5, 198–201 (2012).
Hodson, A. Biogeochemistry of snowmelt in an Antarctic glacial ecosystem. Water Resour. Res. 42, W11406 (2006).
Hood, E. et al. Glaciers as a source of ancient and labile organic matter to the marine environment. Nature 462, 1044–1047 (2009).
Lanoil, B. et al. Bacteria beneath the West Antarctic Ice Sheet. Environ. Microbiol. 11, 609–615 (2009).
Yde, J. C. et al. Basal ice microbiology at the margin of the Greenland ice sheet. Ann. Glaciol. 51, 71–79 (2010).
Wadham, J. L. et al. Potential methane reserves beneath Antarctica. Nature 488, 633–637 (2012).
Barker, J. D., Sharp, M. J., Fitzsimons, S. J. & Turner, R. J. Abundance and dynamics of dissolved organic carbon in glacier systems. Arct. Antarct. Alp. Res. 38, 163–172 (2006).
Bardgett, R. D. et al. Heterotrophic microbial communities use ancient carbon following glacial retreat. Biol. Lett. 3, 487–490 (2007).
Acknowledgements
MS was supported by Danish Research Council grant FNU 10-085274. JŽ acknowledges the support from Marie-Curie ITN NSINK Project No. 215503 and a scholarship of the University of Innsbruck (2011/2/Bio22 140797).
Author information
Authors and Affiliations
Contributions
All authors contributed to writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Stibal, M., Šabacká, M. & Žárský, J. Biological processes on glacier and ice sheet surfaces. Nature Geosci 5, 771–774 (2012). https://doi.org/10.1038/ngeo1611
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo1611
This article is cited by
-
The great melt will shape unprotected ecosystems
Nature (2023)
-
Spatial distribution and stable isotopic composition of invertebrates uncover differences between habitats on the glacier surface in the Alps
Limnology (2023)
-
In situ Nanopore sequencing reveals metabolic characteristics of the Qilian glacier meltwater microbiome
Environmental Science and Pollution Research (2023)
-
Metagenomics reveals global-scale contrasts in nitrogen cycling and cyanobacterial light-harvesting mechanisms in glacier cryoconite
Microbiome (2022)
-
Diversity, distribution, and function of bacteria in the supraglacial region hit by glacial lake outburst flood in northern Pakistan
Environmental Sciences Europe (2022)