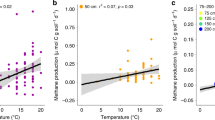
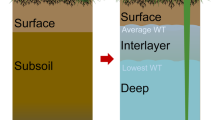
Abstract
Peatlands store vast amounts of organic carbon, amounting to approximately 455 Pg. Carbon builds up in these water-saturated environments owing to the presence of phenolic compounds—which inhibit microbial activity and therefore prevent the breakdown of organic matter. Anoxic conditions limit the activity of phenol oxidase, the enzyme responsible for the breakdown of phenolic compounds. Droughts introduce oxygen into these systems, and the frequency of these events is rising. Here, we combine in vitro manipulations, mesocosm experiments and field observations to examine the impact of drought on peatland carbon loss. We show that drought stimulates bacterial growth and phenol oxidase activity, resulting in a reduction in the concentration of phenolic compounds in peat. This further stimulates microbial growth, causing the breakdown of organic matter and the release of carbon dioxide in a biogeochemical cascade. We further show that re-wetting the peat accelerates carbon losses to the atmosphere and receiving waters, owing to drought-induced increases in nutrient and labile carbon levels, which raise pH and stimulate anaerobic decomposition. We suggest that severe drought, and subsequent re-wetting, could destabilize peatland carbon stocks; understanding this process could aid understanding of interactions between peatlands and other environmental trends, and lead to the development of strategies for increasing carbon stocks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gorham, E. Northern peatlands: Role in the carbon cycle and probable responses to climate warming. Ecol. Appl. 1, 182–195 (1991).
Limpens, J. et al. Peatlands and the carbon cycle: From local processes to global implications—a synthesis. Biogeosciences 5, 1475–1491 (2008).
McLatchey, G. P. & Reddy, K. R. Regulation of organic matter decomposition and nutrient release in a wetland soil. J. Environ. Qual. 27, 1268–1274 (1998).
Laiho, R. Decomposition in peatlands: Reconciling seemingly contrasting results on the impacts of lowered water levels. Soil Biol. Biochem. 38, 2011–2024 (2006).
Freeman, C., Ostle, N. & Kang, H. An enzymic ‘latch’ on a global carbon store. Nature 409, 149 (2001).
Wetzel, R. G. Gradient dominated ecosystems: Sources and regulatory functions of dissolved organic matter in freshwater ecosystems. Hydrobiologia 229, 181–198 (1992).
IPCC Climate Change 2007: Impacts, Adaptation and Vulnerability (Cambridge Univ. Press, 2007).
Worrall, F., Burt, T. P. & Adamson, J. K. Trends in drought frequency—the fate of DOC export from British peatlands. Clim. Change 76, 339–359 (2006).
Fenner, N., Freeman, C. & Reynolds, B. Hydrological effects on the diversity of phenolic degrading bacteria in a peatland: Implications for carbon cycling. Soil Biol. Biochem. 37, 1277–1287 (2005).
Kiss, S., Dragan-Burlarda, M. & Raduleescu, D. Biological significance of enzymes accumulated in soils. Adv. Agron. 27, 25–87 (1975).
Claus, H. Laccases: Structure, reactions, distribution. Micron 35, 93–96 (2004).
Jassey, V. E. J. et al. Experimental climate effect on seasonal variability of polyphenol/phenoloxidase interplay along a narrow fen-bog ecological gradient in Sphagnum fallax. Glob. Change Biol. 17, 2945–2957 (2011).
Freeman, C., Ostle, N. J. & Fenner, N. et al. A regulatory role for phenol oxidase during decomposition in peatlands. Soil Biol. Biochem. 36, 1663–1667 (2004).
Blanchette, R. A. A review of microbial deterioration found in archaeological wood from different environments. Int. Biodeterioration Biodegrad. 46, 189–204 (2000).
Coates, J. D. Diversity and ubiquity of bacteria capable of utilizing humic substances as electron donors for anaerobic respiration. Appl. Environ. Microbiol. 68, 2445–2452 (2002).
Thornton, C. R. Use of monoclonal antibodies to quantify the dynamics of α-galactosidase and endo-1,4-β-glucanase production by Trichoderma hamatum during saprotrophic growth and sporulation in peat. Environ. Microbiol. 7, 737–749 (2005).
Knorr, K-H. & Blodau, C. Impact of experimental drought and rewetting on redox transformations and methanogenesis in mesocosms of a northern fen soil. Soil Biol. Biochem. 41, 1187–1198 (2009).
Stainier, R. Y. et al. The Microbial World 5th edn,78–101 (Prentice-Hall, 1986).
Fontaine, S. et al. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450, 8 (2007).
Freeman, C. & Fenner, N. et al. Export of dissolved organic carbon from peatlands under elevated carbon dioxide levels. Nature 430, 195–198 (2004).
Williams, C. J. et al. Phenol oxidase activity in peatlands in New York state: Response to summer drought and peat type. Wetlands 20, 416–421 (2000).
Pind, A., Freeman, C. & Lock, M. A. Enzymic degradation of phenolic materials in peatlands—measurement of phenol oxidase activity. Plant Soil 159, 227–231 (1994).
Smolders, A. J. P. Internal eutrophication: How it works and what to do about it—a review. Chem. Ecol. 22, 93–111 (2006).
Monteith, D. T. Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 450, 537–541 (2007).
Toberman, H. et al. Summer drought effects upon soil and litter extracellular phenol oxidase activity and soluble carbon release in an upland Calluna heathland. Soil Biol. Biochem. 40, 1519–1532 (2008).
Worrall, F., Burt, T. & Adamson, J. Fluxes of dissolved carbon dioxide and inorganic carbon from an upland peat catchment: Implications for soil respiration. Biogeochemistry 73, 515–539 (2005).
Jackson, B. E. & McInerny, M. J. Anaerobic microbial metabolism can proceed close to thermodynamic limits. Nature 415, 454–456 (2002).
Alm, J. et al. Carbon balance of a boreal bog during a year with an exceptionally dry summer. Ecology 80, 161–174 (1999).
Griffis, T. J., Rouse, W. R. & Waddington, J. M. Interannual variability of net ecosystem CO2 exchange at a subartic fen. Glob. Biogeochem. Cycles 14, 1109–1121 (2000).
Roulet, N. T. et al. Contemporary carbon balance and late Holocene carbon accumulation in a northern peatland. Glob. Change Biol. 13, 397–411 (2007).
Reiche, M. et al. (2009), Impact of manipulated drought and heavy rainfall events on peat mineralization processes and source–sink functions of an acidic fen. J. Geophys. Res. 114, G02021.
Masing, V. et al. in Wetlands and Shallow Continental Water Bodies Vol. 1 (SPB, 1990).
Acknowledgements
N.F. and C.F. acknowledge funding from the Natural Environment Research Council, the Royal Society, the Leverhulme Trust, EU-FP7-ERA-ENVHEALTH and the Bioscience Environment and Agricultural Alliance (Wales, UK).
Author information
Authors and Affiliations
Contributions
N.F. wrote the paper and carried out the experiments. C.F. co-wrote the paper and conceived the experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 671 kb)
Rights and permissions
About this article
Cite this article
Fenner, N., Freeman, C. Drought-induced carbon loss in peatlands. Nature Geosci 4, 895–900 (2011). https://doi.org/10.1038/ngeo1323
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo1323
This article is cited by
-
Climate warming and elevated CO2 alter peatland soil carbon sources and stability
Nature Communications (2023)
-
Responses of the content and spectral characteristics of dissolved organic matter in intercropping soil to drought in northeast China
Plant and Soil (2023)
-
Moisture-dependent response of soil carbon mineralization to temperature increases in a karst wetland on the Yunnan-Guizhou Plateau
Environmental Science and Pollution Research (2023)
-
Anthropogenic impacts on lowland tropical peatland biogeochemistry
Nature Reviews Earth & Environment (2022)
-
Asynchronous responses of microbial CAZymes genes and the net CO2 exchange in alpine peatland following 5 years of continuous extreme drought events
ISME Communications (2022)