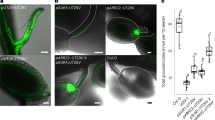
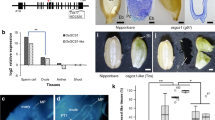
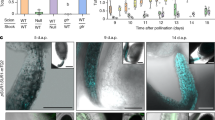
Abstract
Carbohydrate import into seeds directly determines seed size and must have been increased through domestication. However, evidence of the domestication of sugar translocation and the identities of seed-filling transporters have been elusive. Maize ZmSWEET4c, as opposed to its sucrose-transporting homologs, mediates transepithelial hexose transport across the basal endosperm transfer layer (BETL), the entry point of nutrients into the seed, and shows signatures indicative of selection during domestication. Mutants of both maize ZmSWEET4c and its rice ortholog OsSWEET4 are defective in seed filling, indicating that a lack of hexose transport at the BETL impairs further transfer of sugars imported from the maternal phloem. In both maize and rice, SWEET4 was likely recruited during domestication to enhance sugar import into the endosperm.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chourey, P.S., Jain, M., Li, Q.-B. & Carlson, S.J. Genetic control of cell wall invertases in developing endosperm of maize. Planta 223, 159–167 (2006).
Wang, E. et al. Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nat. Genet. 40, 1370–1374 (2008).
Lalonde, S., Wipf, D. & Frommer, W.B. Transport mechanisms for organic forms of carbon and nitrogen between source and sink. Annu. Rev. Plant Biol. 55, 341–372 (2004).
Bihmidine, S., Hunter, C.T.I., Johns, C.E., Koch, K.E. & Braun, D.M. Regulation of assimilate import into sink organs: update on molecular drivers of sink strength. Front. Plant Sci. 4, 177 (2013).
Glémin, S. & Battaillon, T. A comparative view of the evolution of grasses under domestication. New Phytol. 183, 273–290 (2009).
Peng, J. et al. 'Green revolution' genes encode mutant gibberellin response modulators. Nature 400, 256–261 (1999).
Sekhon, R.S. et al. Genome-wide atlas of transcription during maize development. Plant J. 66, 553–563 (2011).
Li, G. et al. Temporal patterns of gene expression in developing maize endosperm identified through transcriptome sequencing. Proc. Natl. Acad. Sci. USA 111, 7582–7587 (2014).
Davidson, R.M., Hansey, C.N. & Gowda, M. Utility of RNA sequencing for analysis of maize reproductive transcriptomes. Plant Genome 4, 191–203 (2011).
Hufford, M.B. et al. Comparative population genomics of maize domestication and improvement. Nat. Genet. 44, 808–811 (2012).
Chen, L.-Q. et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468, 527–532 (2010).
Chen, L.-Q. et al. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 335, 207–211 (2012).
Lin, I.W. et al. Nectar secretion requires sucrose phosphate synthases and the sugar transporter SWEET9. Nature 508, 546–549 (2014).
Lemmon, Z.H. et al. The role of cis regulatory evolution in maize domestication. PLoS Genet. 10, e1004745 (2014).
Chourey, P.S., Li, Q.-B. & Cevallos-Cevallos, J. Pleiotropy and its dissection through a metabolic gene Miniature1 (Mn1) that encodes a cell wall invertase in developing seeds of maize. Plant Sci. 184, 45–53 (2012).
Flint-Garcia, S.A., Bodnar, A. & Scott, M.P. Wide variability in seed characteristics, kernel quality, and zein profiles among diverse maize inbreds, landraces, and teosinte. Theor. Appl. Genet. 119, 1129–1142 (2009).
Sosso, D., Javelle, M. & Rogowsky, P.M. in Advances in Maize Vol. 3 (eds. Prioul, J.L., Thevenot, C. & Molnar, T.) 163–188 (Society for Experimental Biology, 2011).
Lucas, W.J. et al. The plant vascular system: evolution, development and functions. J. Integr. Plant Biol. 55, 294–388 (2013).
Chen, L.-Q. et al. Embryo nutrition by a cascade of sequentially expressed sucrose transporters in the seed coat. Plant Cell 27, 607–619 (2015).
Shannon, J.C. Movement of 14C-labeled assimilates into kernels of Zea mays L.: I. pattern and rate of sugar movement. Plant Physiol. 49, 198–202 (1972).
Schmalstig, J.G. & Hitz, W.D. Transport and metabolism of a sucrose analog (1′-fluorosucrose) into Zea mays L. endosperm without invertase hydrolysis. Plant Physiol. 85, 902–905 (1987).
Cheng, W.H., Taliercio, E.W. & Chourey, P.S. The Miniature1 seed locus of maize encodes a cell wall invertase required for normal development of endosperm and maternal cells in the pedicel. Plant Cell 8, 971–983 (1996).
Koch, K. Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 7, 235–246 (2004).
Xiong, Y., Li, Q.-B., Kang, B.-H. & Chourey, P.S. Discovery of genes expressed in basal endosperm transfer cells in maize using 454 transcriptome sequencing. Plant Mol. Biol. Rep. 29, 835–847 (2011).
Klemens, P.A.W. et al. Overexpression of the vacuolar sugar carrier AtSWEET16 modifies germination, growth, and stress tolerance in Arabidopsis. Plant Physiol. 163, 1338–1352 (2013).
Guo, W.J. et al. SWEET17, a facilitative transporter, mediates fructose transport across the tonoplast of Arabidopsis roots and leaves. Plant Physiol. 164, 777–789 (2014).
Tao, Y. et al. Structure of a eukaryotic SWEET transporter in a homotrimeric complex. Nature doi:10.1038/nature15391 (19 October 2015).
Chen, H.-Y. et al. The Arabidopsis vacuolar sugar transporter SWEET2 limits carbon sequestration from roots and restricts Pythium infection. Plant J. 83, 1046–1058 (2015).
Wieczorke, R. et al. Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett. 464, 123–128 (1999).
Kellett, G.L., Brot-Laroche, E., Mace, O.J. & Leturque, A. Sugar absorption in the intestine: the role of GLUT2. Annu. Rev. Nutr. 28, 35–54 (2008).
Zheng, Y. & Wang, Z. Current opinions on endosperm transfer cells in maize. Plant Cell Rep. 29, 935–942 (2010).
Thompson, R.D., Hueros, G., Becker, H.A. & Maitz, M. Development and functions of seed transfer cells. Plant Sci. 160, 775–783 (2001).
Wardini, T., Talbot, M.J., Offler, C.E. & Patrick, J.W. Role of sugars in regulating transfer cell development in cotyledons of developing Vicia faba seeds. Protoplasma 230, 75–88 (2007).
Gómez, E. et al. The maize transcription factor Myb-related protein-1 is a key regulator of the differentiation of transfer cells. Plant Cell 21, 2022–2035 (2009).
Barrero, C. et al. The promoter of ZmMRP-1, a maize transfer cell–specific transcriptional activator, is induced at solute exchange surfaces and responds to transport demands. Planta 229, 235–247 (2009).
Borisjuk, L. et al. Seed development and differentiation: a role for metabolic regulation. Plant Biol. 6, 375–386 (2004).
Eom, J.S. et al. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr. Opin. Plant Biol. 25, 53–62 (2015).
Huang, X. et al. A map of rice genome variation reveals the origin of cultivated rice. Nature 490, 497–501 (2012).
McCarty, D.R. et al. Steady-state transposon mutagenesis in inbred maize. Plant J. 44, 52–61 (2005).
McCarty, D.R. et al. Mu-seq: sequence-based mapping and identification of transposon induced mutations. PLoS ONE 8, e77172 (2013).
Hunter, C.T. et al. Phenotype to genotype using forward-genetic Mu-seq for identification and functional classification of maize mutants. Front. Plant Sci. 4, 545 (2014).
Hufford, M.B. et al. Comparative population genomics of maize domestication and improvement. Nat. Genet. 44, 808–811 (2012).
Thornton, K. et al. Libsequence: a C. class library for evolutionary genetic analysis. Bioinformatics 19, 2325–2327 (2003).
Piperno, D.R. et al. Starch grain and phytolith evidence for early ninth millennium B.P. maize from the Central Balsas River Valley, Mexico. Proc. Natl. Acad. Sci. USA 106, 5019–5024 (2009).
Wright, S.I. et al. The effects of artificial selection on the maize genome. Science 308, 1310–1314 (2005).
Hudson, R.R. et al. Generating samples under a Wright-Fisher neutral model of genetic variation. Bioinformatics 18, 337–338 (2002).
Hudson, R.R. et al. A statistical test for detecting geographic subdivision. Mol. Biol. Evol. 9, 138–151 (1992).
Danecek, P. et al. The variant call format and VCFtools. Bioinformatics 27, 2156–2158 (2011).
Jackson, D.P. in Molecular Plant Pathology: A Practical Approach (eds. Bowles, D.J., Gurr, S.J. & McPhereson, M.) 163–174 (Oxford University Press, 1991).
Wang, C. et al. Isolation and characterization of expressed sequence tags (ESTs) from cambium tissue of birch (Betula platyphylla Suk). Plant Mol. Biol. Rep. 28, 438–449 (2010).
De Oliveira, R.R. et al. In silico and quantitative analyses of MADS-Box genes in Coffea arabica. Plant Mol. Biol. Rep. 28, 460–472 (2010).
Hou, B.H. et al. Optical sensors for monitoring dynamic changes of metabolite levels in mammalian cells. Nat. Protoc. 6, 1818–1833 (2011).
Zhou, J. et al. Gene targeting by the TAL effector PthXo2 reveals cryptic resistance gene for bacterial blight of rice. Plant J. 82, 632–643 (2015).
Cheng, W.H. & Chourey, P.S. Genetic evidence that invertase-mediated release of hexoses is critical for appropriate carbon partitioning and normal seed development in maize. Theor. Appl. Genet. 98, 485–495 (1999).
Li, T. et al. TALEN utilization in rice genome modifications. Methods 69, 9–16 (2014).
Li, T. et al. High efficiency TALEN-based gene editing produces disease resistant rice. Nat. Biotechnol. 30, 390–392 (2012).
Bleckmann, A. et al. Stem cell signaling in Arabidopsis requires CRN to localize CLV2 to the plasma membrane. Plant Physiol. 152, 166–176 (2010).
Dupreet, P. et al. Expression of photosynthesis gene-promoter fusions in leaf epidermal cells of transgenic tobacco plants. Plant J. 1, 115–120 (1991).
Zhang, Y. et al. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 7, 30 (2011).
Jefferson, R.A., Kavanagh, T.A. & Bevan, M.W. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, 3901–3907 (1987).
Scheet, P. & Stephens, M. A fast and flexible statistical model for large-scale population genotype data: applications to inferring missing genotypes and haplotypic phase. Am. J. Hum. Genet. 78, 629–644 (2006).
Paradis, E. pegas: an R package for population genetics with an integrated-modular approach. Bioinformatics 26, 419–420 (2010).
Acknowledgements
We are grateful to D. Ehrhardt and H. Cartwright for confocal microscopy. For the rice experiments, we are grateful to T. Li for constructing the TALEN vector targeting OsSWEET4, B. Liu for rice transgenics, C. Ji for the isolation and transfection of rice protoplasts in the laboratory of B.Y. and X. Li for help with domestication analysis of OsSWEET4. We thank M. Greenfield, A. Grimault and K.M. Wong for plant care, Y. Gong for the yeast complementation assay, M. Evans for providing teosinte plant material and C. Stefan for renaming ZmSWEET4 with “c” for her initial. Work performed on maize in the laboratory of W.B.F. was made possible by support from the Office of Basic Energy Sciences of the US Department of Energy under grant DE-FG02-04ER15542, and work on rice was supported by the National Science Foundation under grant IOS-1258018 (B.Y. and W.B.F.); the other laboratories were supported by the National Science Foundation (IOS-1116561 to D.R.M. and K.E.K., IOS-1025976 to K.E.K. and IOS-1238014 to J.R.-I.), as well as USDA-NIFA 2010-04228 (K.E.K., D.R.M. and M.S.).
Author information
Authors and Affiliations
Contributions
D.S., P.S.C., P.M.R., J.R.-I., B.Y. and W.B.F. conceived and designed experiments. D.S., D.L., Q.-B.L., J.S., J.Y., G.G., M.S., K.E.K., D.R.M. and J.R.-I. performed experiments. D.S., P.S.C., J.R.-I., B.Y., K.E.K., J.Y., D.R.M. and W.B.F. analyzed the data. D.S. and W.B.F. wrote the manuscript, and J.S., M.S., K.E.K., D.R.M., P.S.C., P.M.R., J.R.-I. and B.Y. revised it.
Corresponding author
Ethics declarations
Competing interests
Patent applications regarding the use of SWEET genes have been filed.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–16 and Supplementary Tables 1–4. (PDF 3171 kb)
Rights and permissions
About this article
Cite this article
Sosso, D., Luo, D., Li, QB. et al. Seed filling in domesticated maize and rice depends on SWEET-mediated hexose transport. Nat Genet 47, 1489–1493 (2015). https://doi.org/10.1038/ng.3422
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3422
This article is cited by
-
Genome-wide identification of SWEET genes reveals their roles during seed development in peanuts
BMC Genomics (2024)
-
Plasma membrane-localized hexose transporter OsSWEET1b, affects sugar metabolism and leaf senescence
Plant Cell Reports (2024)
-
A translatome-transcriptome multi-omics gene regulatory network reveals the complicated functional landscape of maize
Genome Biology (2023)
-
Multi-omic characterization of the maize GPI synthesis mutant gwt1 with defects in kernel development
BMC Plant Biology (2023)
-
Genome-wide identification, characterization and transcriptional profile of the SWEET gene family in Dendrobium officinale
BMC Genomics (2023)