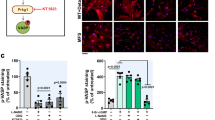
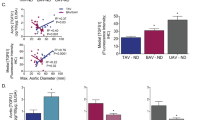
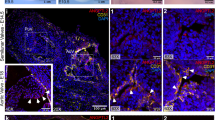
Abstract
Loeys-Dietz syndrome (LDS) associates with a tissue signature for high transforming growth factor (TGF)-β signaling but is often caused by heterozygous mutations in genes encoding positive effectors of TGF-β signaling, including either subunit of the TGF-β receptor or SMAD3, thereby engendering controversy regarding the mechanism of disease. Here, we report heterozygous mutations or deletions in the gene encoding the TGF-β2 ligand for a phenotype within the LDS spectrum and show upregulation of TGF-β signaling in aortic tissue from affected individuals. Furthermore, haploinsufficient Tgfb2+/− mice have aortic root aneurysm and biochemical evidence of increased canonical and noncanonical TGF-β signaling. Mice that harbor both a mutant Marfan syndrome (MFS) allele (Fbn1C1039G/+) and Tgfb2 haploinsufficiency show increased TGF-β signaling and phenotypic worsening in association with normalization of TGF-β2 expression and high expression of TGF-β1. Taken together, these data support the hypothesis that compensatory autocrine and/or paracrine events contribute to the pathogenesis of TGF-β–mediated vasculopathies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Moustakas, A. & Heldin, C.H. The regulation of TGFβ signal transduction. Development 136, 3699–3714 (2009).
Rahimi, R.A. & Leof, E.B. TGF-β signaling: a tale of two responses. J. Cell. Biochem. 102, 593–608 (2007).
Dietz, H.C. et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 352, 337–339 (1991).
Isogai, Z. et al. Latent transforming growth factor β–binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J. Biol. Chem. 278, 2750–2757 (2003).
Chaudhry, S.S. et al. Fibrillin-1 regulates the bioavailability of TGFβ1. J. Cell Biol. 176, 355–367 (2007).
Loeys, B.L. et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 37, 275–281 (2005).
Mizuguchi, T. et al. Heterozygous TGFBR2 mutations in Marfan syndrome. Nat. Genet. 36, 855–860 (2004).
van de Laar, I.M. et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat. Genet. 43, 121–126 (2011).
Renard, M. et al. Altered TGFβ signaling and cardiovascular manifestations in patients with autosomal recessive cutis laxa type I caused by fibulin-4 deficiency. Eur. J. Hum. Genet. 18, 895–901 (2010).
Gomez, D. et al. Syndromic and non-syndromic aneurysms of the human ascending aorta share activation of the Smad2 pathway. J. Pathol. 218, 131–142 (2009).
Habashi, J.P. et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 312, 117–121 (2006).
Holm, T.M. et al. Noncanonical TGFβ signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science 332, 358–361 (2011).
Cohn, R.D. et al. Angiotensin II type 1 receptor blockade attenuates TGF-β–induced failure of muscle regeneration in multiple myopathic states. Nat. Med. 13, 204–210 (2007).
Ng, C.M. et al. TGF-β–dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J. Clin. Invest. 114, 1586–1592 (2004).
Arteaga-Solis, E. et al. Regulation of limb patterning by extracellular microfibrils. J. Cell Biol. 154, 275–281 (2001).
Loeys, B.L. et al. Aneurysm syndromes caused by mutations in the TGF-β receptor. N. Engl. J. Med. 355, 788–798 (2006).
Choudhary, B. et al. Absence of TGFβ signaling in embryonic vascular smooth muscle leads to reduced lysyl oxidase expression, impaired elastogenesis, and aneurysm. Genesis 47, 115–121 (2009).
Langlois, D. et al. Conditional inactivation of TGF-β type II receptor in smooth muscle cells and epicardium causes lethal aortic and cardiac defects. Transgenic Res. 19, 1069–1082 (2010).
Lindsay, M.E. & Dietz, H.C. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature 473, 308–316 (2011).
Sunyaev, S. et al. Prediction of deleterious human alleles. Hum. Mol. Genet. 10, 591–597 (2001).
Ng, P.C. & Henikoff, S. Predicting deleterious amino acid substitutions. Genome Res. 11, 863–874 (2001).
Schwarz, J.M., Rodelsperger, C., Schuelke, M. & Seelow, D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat. Methods 7, 575–576 (2010).
1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature 467, 1061–1073 (2010).
Maleszewski, J.J., Miller, D.V., Lu, J., Dietz, H.C. & Halushka, M.K. Histopathologic findings in ascending aortas from individuals with Loeys-Dietz syndrome (LDS). Am. J. Surg. Pathol. 33, 194–201 (2009).
Bartram, U. et al. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-β2–knockout mice. Circulation 103, 2745–2752 (2001).
McKusick, V.A. The cardiovascular aspects of Marfan′s syndrome: a heritable disorder of connective tissue. Circulation 11, 321–342 (1955).
Judge, D.P. et al. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J. Clin. Invest. 114, 172–181 (2004).
Habashi, J.P. et al. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science 332, 361–365 (2011).
Neptune, E.R. et al. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 33, 407–411 (2003).
Iwata, J. et al. Modulation of noncanonical TGF-β signaling prevents cleft palate in Tgfbr2 mutant mice. J. Clin. Invest. 122, 873–885 (2012).
Matt, P. et al. Circulating transforming growth factor-β in Marfan syndrome. Circulation 120, 526–532 (2009).
Acknowledgements
This study was supported in part by funding from the Fund for Scientific Research, Flanders (FWO; Belgium) (G.0458.09 and G.0221.12); a European Grant Fighting Aneurysmal Disease (EC-FP7); the Special Research Fund of Ghent University (BOF10/GOA/005); the US National Institutes of Health (RO1- AR41135 and PO1-AR049698 to H.C.D., 5RC1HL100021-02 to J.V.E. and H.C.D. and an Institutional Clinical and Translational Science Award 1U54RR023561-01A1 to J.V.E.); the National Marfan Foundation; the Smilow Center for Marfan Syndrome Research; the Howard Hughes Medical Institute; the Freudmann Fund for Research in Ehlers Danlos Syndrome and Related Disorders; and the Baylor-Hopkins Center for Mendelian Genetics (1U54HG006542). B.L.L. is senior clinical investigator of the Fund for Scientific Research, Flanders (Belgium); N.A.B. is supported by the Aneurysmal Pathology Foundation; D.S. is supported by a PhD grant from the Agency for Innovation by Science and Technology (IWT); E.G. is supported by a fellowship from the Helen Hay Whitney Foundation; J.J.D. is supported by the McKusick Fellowship of the National Marfan Foundation; and M.E.L. is supported by an NHLBI K08 Award (HL107738-01) and by a Fellow-to-Faculty Award from the National Marfan Foundation.
Author information
Authors and Affiliations
Contributions
M.E.L., H.C.D., D.S., L.V.L. and B.L.L. conceived of the study and designed all experiments. M.E.L., D.S., L.V.L., H.C.D. and B.L.L. wrote the manuscript. D.S., M.H.Y. and N.A.B. performed microarray experiments and mutation analysis. J.J.D. performed protein blotting experiments. E.G. performed RT-PCR analysis of mouse aortas. J.F.-B. and J.V.E. performed serum TGF-β ligand analysis. E.K.F. performed, interpreted and produced multidetector-computed tomography images. Y.C. performed animal husbandry, genotyping and aorta dissections. L.M. performed IHC on human and mouse samples. D.B. performed all mouse echocardiograms. M.J.E.K., G.O., B.-M.A., E.M.H.F.B., J.T., A.C.B., N.C., G.R.M., H.G.B. and P.H.B. contributed patient material and clinical and pedigree data and revised the manuscript. A.F.E. and H.P.L. contributed to the whole-exome sequencing initiative.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1 and 2 and Supplementary Figures 1–7 (PDF 6608 kb)
Rights and permissions
About this article
Cite this article
Lindsay, M., Schepers, D., Bolar, N. et al. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat Genet 44, 922–927 (2012). https://doi.org/10.1038/ng.2349
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2349
This article is cited by
-
The roles and regulatory mechanisms of TGF-β and BMP signaling in bone and cartilage development, homeostasis and disease
Cell Research (2024)
-
Severity of Autism Spectrum Disorder Symptoms Associated with de novo Variants and Pregnancy-Induced Hypertension
Journal of Autism and Developmental Disorders (2024)
-
Diagnosis and treatment of cardiovascular disease in patients with heritable connective tissue disorders or heritable thoracic aortic diseases
Cardiovascular Intervention and Therapeutics (2024)
-
Genome-wide association study of varicose veins identifies a protective missense variant in GJD3 enriched in the Finnish population
Communications Biology (2023)
-
The mechanism and therapy of aortic aneurysms
Signal Transduction and Targeted Therapy (2023)