Abstract
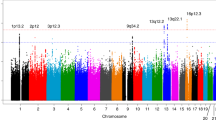
Pancreatic cancer has the lowest survival rate among human cancers, and there are no effective markers for its screening and early diagnosis. To identify genetic susceptibility markers for this cancer, we carried out a genome-wide association study on 981 individuals with pancreatic cancer (cases) and 1,991 cancer-free controls of Chinese descent using 666,141 autosomal SNPs. Promising associations were replicated in an additional 2,603 pancreatic cancer cases and 2,877 controls recruited from 25 hospitals in 16 provinces or cities in China. We identified five new susceptibility loci at chromosomes 21q21.3, 5p13.1, 21q22.3, 22q13.32 and 10q26.11 (P = 2.24 × 10−13 to P = 4.18 × 10−10) in addition to 13q22.1 previously reported in populations of European ancestry. These results advance our understanding of the development of pancreatic cancer and highlight potential targets for the prevention or treatment of this cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Li, D., Xie, K., Wolff, R. & Abbruzzese, J.L. Pancreatic cancer. Lancet 363, 1049–1057 (2004).
Klein, A.P. et al. Familial pancreatic cancer. Cancer J. 7, 266–273 (2001).
Petersen, G.M. et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat. Genet. 42, 224–228 (2010).
Amundadottir, L. et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat. Genet. 41, 986–990 (2009).
Low, S.K. et al. Genome-wide association study of pancreatic cancer in Japanese population. PLoS ONE 5, e11824 (2010).
Wolpin, B.M. et al. ABO blood group and the risk of pancreatic cancer. J. Natl. Cancer Inst. 101, 424–431 (2009).
Wolpin, B.M. et al. Pancreatic cancer risk and ABO blood group alleles: results from the pancreatic cancer cohort consortium. Cancer Res. 70, 1015–1023 (2010).
Iodice, S. et al. ABO blood group and cancer. Eur. J. Cancer 46, 3345–3350 (2010).
Ben, Q., Wang, K., Yuan, Y. & Li, Z. Pancreatic cancer incidence and outcome in relation to ABO blood groups among Han Chinese patients: a case-control study. Int. J. Cancer 128, 1179–1186 (2011).
Nakao, M. et al. ABO blood group alleles and the risk of pancreatic cancer in a Japanese population. Cancer Sci. 102, 1076–1080 (2011).
Sun, J. et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 21, 5216–5224 (2002).
Reichard, J.F., Sartor, M.A. & Puga, A. BACH1 is a specific repressor of HMOX1 that is inactivated by arsenite. J. Biol. Chem. 283, 22363–22370 (2008).
Vijayan, V., Mueller, S., Baumgart-Vogt, E. & Immenschuh, S. Heme oxygenase-1 as a therapeutic target in inflammatory disorders of the gastrointestinal tract. World J. Gastroenterol. 16, 3112–3119 (2010).
Zhu, X. et al. Heme oxygenase-1 system and gastrointestinal tumors. World J. Gastroenterol. 16, 2633–2637 (2010).
Harusato, A. et al. Inhibition of Bach1 ameliorates indomethacin-induced intestinal injury in mice. J. Physiol. Pharmacol. 60 (suppl. 7), 149–154 (2009).
Iida, A. et al. Bach1 deficiency ameliorates hepatic injury in a mouse model. Tohoku J. Exp. Med. 217, 223–229 (2009).
Fazili, Z. et al. Loss of Disabled-2 is an early step in ovarian tumorigenicity. Oncogene 18, 3104–3113 (1999).
He, J., Smith, E.R. & Xu, X.X. Disabled-2 exerts its tumor suppressor activity by uncoupling c-Fos expression and MAP kinase activation. J. Biol. Chem. 276, 26814–26818 (2001).
Hannigan, A. et al. Epigenetic downregulation of human disabled homolog 2 switches TGF-β from a tumor suppressor to a tumor promoter. J. Clin. Invest. 120, 2842–2857 (2010).
Huang, Y. et al. Doc-2/hDab2 expression is up-regulated in primary pancreatic cancer but reduced in metastasis. Lab. Invest. 81, 863–873 (2001).
Yang, D.H. et al. Disabled-2 heterozygous mice are predisposed to endometrial and ovarian tumorigenesis and exhibit sex-biased embryonic lethality in a p53-null background. Am. J. Pathol. 169, 258–267 (2006).
Prunier, C. & Howe, P.H. Disabled-2 (Dab2) is required for transforming growth factor β-induced epithelial to mesenchymal transition (EMT). J. Biol. Chem. 280, 17540–17548 (2005).
Chaudhury, A. et al. TGF-β-mediated phosphorylation of hnRNP E1 induced EMT via transcript-selective translational induction of Dab2 and ILEI. Nat. Cell Biol. 12, 286–293 (2010).
Jiang, Y., Lou, W. & Howe, P.H. Dab2 stabilizes axin and attenuates Wnt/β-catenin signaling by preventing protein phosphatase 1(PP1)-axin interactions. Oncogene 28, 2999–3007 (2009).
Pasca di Magliano, M. et al. Common activation of canonical Wnt signaling in pancreatic adenocarcinoma. PLoS ONE 2, e1155 (2007).
Heiser, P.W. et al. Stabilization of β-catenin induces pancreas tumor formation. Gastroenterology 135, 1288–1300 (2008).
Emami, S. et al. Trefoil factor family (TFF) peptide cancer progression. Peptides 25, 885–898 (2004).
Ohshio, G. et al. Differential expression of human spasmolytic polypeptide (trefoil factor family-2) in pancreatic carcinoma, ampullary carcinoma, and mucin-producing tumors of the pancreas. Dig. Dis. Sci. 45, 659–664 (2000).
Argani, P. et al. Discovery of new markers of cancer through serial analysis of gene expression: prostate stem cell antigen is overexpressed in pancreatic adenocarcinoma. Cancer Res. 61, 4320–4324 (2001).
Yeh, T.S. et al. Characterisation of oestroge receptor, progesterone receptor, trefoil factor 1, and epidermal growth factor and its receptor in pancreatic cystic neoplasms and pancreatic ductal adenocarcinoma. Gut 51, 712–716 (2002).
Dubeykovskaya, Z., Dubeykovskiy, A., Solal-Cohen, J. & Wang, T.C. Secreted trefoil factor 2 activates the CXCR4 receptor in epithelial and lymphocytic cancer cell lines. J. Biol. Chem. 284, 3650–3662 (2009).
Díaz de Ståhl, T. et al. Chromosome 22 tiling-path array-CGH analysis identifies germ-line- and tumor-specific aberration in patients with glioblastoma multiforme. Genes Chromosom. Cancer 44, 161–169 (2005).
Gu, W. et al. The prolactin-releasing peptide receptor (GPR10) regulates body weight homeostasis in mice. J. Mol. Neurosci. 22, 93–103 (2004).
Bjursell, M. et al. GPR10 deficiency in mice results in altered energy expenditure and obesity. Biochem. Biophys. Res. Commun. 363, 633–638 (2007).
Li, D. et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. J. Am. Med. Assoc. 301, 2553–2562 (2009).
Giovannucci, E. & Michaud, D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology 132, 2208–2225 (2007).
Jiao, L. et al. Body mass index, effect modifiers, and risk of pancreatic cancer: a pooled study of seven prospective cohorts. Cancer Causes Control 21, 1305–1314 (2010).
Yang, M. et al. Functional variants in cell death pathway genes and risk of pancreatic cancer. Clin. Cancer Res. 14, 3230–3236 (2008).
Zhao, D. et al. Interaction of cyclooxygenase-2 variants and smoking in pancreatic cancer: a possible role of nucleophosmin. Gastroenterology 136, 1659–1668 (2009).
Dixon, A.L. et al. A genome-wide association study of global gene expression. Nat. Genet. 39, 1202–1207 (2007).
Leek, J.T. & Storey, J.D. Capturing heterogeneity in gene expression studies by surrogate variable analysis. PLoS Genet. 3, 1724–1735 (2007).
Abecasis, G.R., Cherny, S.S., Cookson, W.O. & Cardon, L.R. Merlin–rapid analysis of dense genetic maps using sparse gene flow trees. Nat. Genet. 30, 97–101 (2002).
Chen, W.M. & Abecasis, G.R. Family-based association tests for genomewide association scans. Am. J. Hum. Genet. 81, 913–926 (2007).
Acknowledgements
This work was supported by grants from the National High-Tech Research and Development Program of China (2009AA022706) and the National Natural Science Foundation of China (81021061) to D.L. We thank L. Liang for help in eQTL analysis. We also thank the subjects and the surgeons who recruited them; M. Yang, L. Wang, X. Wang, C. Yang, L. Du, J. Li, F. Qi, X. Song and L. Zeng.
Author information
Authors and Affiliations
Contributions
D.L. conceived, designed and oversaw the study, obtained financial support, interpreted the results and wrote parts of and synthesized the paper. C. Wu managed the project, oversaw laboratory and statistical analyses and drafted the initial manuscript. Chengfeng Wang designed the study and oversaw pancreatic cancer patient recruitment. X.M. designed the study and carried out statistical analyses, subject recruitment and sample preparation of Zhejiang samples. L.H., K.Z., J.C. and J.X. prepared samples and did TaqMan genotyping. X.C., D.Y., Y.L., W.T. and P.Z. recruited subjects from Beijing, Shandong, Sichuan and Chongqing. Various authors recruited subjects and samples from Shanghai (G.J., G.C., X.Y., Z.L., L.L., M.S. and J.G.), Liaoning (X.Y., W.D., W.C. and Y.M.), Jiangsu (Z.H., H.S. and Yifeng Zhou), Fujian (Yongjian Zhou, Y.C., S.Z. and X. Zheng), Hunan (C.Z.), Hubei (Chunyou Wang and T.W.), Hebei (X. Zhang and Y.J.) and Hong Kong (X.W. and S.T.C.).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 and Supplementary Tables 1–7 (PDF 641 kb)
Rights and permissions
About this article
Cite this article
Wu, C., Miao, X., Huang, L. et al. Genome-wide association study identifies five loci associated with susceptibility to pancreatic cancer in Chinese populations. Nat Genet 44, 62–66 (2012). https://doi.org/10.1038/ng.1020
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.1020
This article is cited by
-
Risk SNP in a transcript of RP11-638I2.4 increases lncRNA–YY1 interaction and pancreatic cancer susceptibility
Archives of Toxicology (2023)
-
Improving the prognosis of pancreatic cancer: insights from epidemiology, genomic alterations, and therapeutic challenges
Frontiers of Medicine (2023)
-
Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors
Nature Reviews Gastroenterology & Hepatology (2021)
-
Identification of genetic variants in m6A modification genes associated with pancreatic cancer risk in the Chinese population
Archives of Toxicology (2021)
-
Pancreatic cancer pathology viewed in the light of evolution
Cancer and Metastasis Reviews (2021)