Abstract
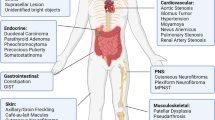
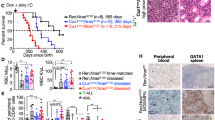
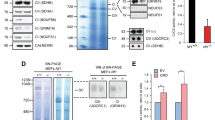
Individuals with neurofibromatosis type 1 (NF1) are predisposed to certain cancers including juvenile chronic myelogenous leukaemia (JCML). The NF1 tumour-suppressor gene encodes a protein (neurofibromin) that accelerates GTP hydrolysis on Ras proteins. Here we show that primary leukaemic cells from children with NF1 show a selective decrease in NF1-like GTPase activating protein (GAP) activity for Ras but retain normal cellular GAP activity. Leukaemic cells also show an elevated percentage of Ras in the GTP-bound conformation. JCML cells are hypersensitive to granulocyte-macrophage colony stimulating factor (GM-CSF), and we observed a similar pattern of aberrant growth in haematopoietic cells from Nf1−/− mouse embryos. These data define a specific role for neurofibromin in negatively regulating GM-CSF signaling through Ras in haematopoietic cells and they suggest that hypersensitivity to GM-CSF may be a primary event in the development of JCML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Riccardi, V.M. & Eichner, J.E. Neurofibromatosis (Johns Hopkins University Press, Baltimore- USA, 1986).
Bader, J.L. & Miller, R.W. Neurofibromatosis and childhood leukemia. J. Pediat. 92, 925–929 (1978).
Shannon, K.M. et al. Monosomy 7 myeloproliferative disease in children with neurofibromatosis, type 1: epidemiology and molecular analysis. Blood 79, 1311–1318 (1992).
Gadner, H. & Haas, O.A. Experience in pediatric myelodysplastic syndromes. Hemat. Clin. N.A. 6, 655–672 (1992).
Skuse, G.R., Kosciolek, B.A. & Rowley, P.T. Molecular genetic analysis of tumors in von Recklinghausen neurofibromatosis: loss of heterozygosity for chromosome 17. Genes Chrom. Cancer 1, 36–41 (1989).
Menon, A.G. et al. Chromosome 17p deletions and p53 gene mutations associated with the formation of malignant neurofibrosarcomas in von Recklinghausen neurofibromatosis. Proc. Natl. Acad. Sci. USA 87, 5435–5439 (1990).
Glover, T.W. et al. Molecular and cytogenetic analysis of tumors in von Recklinghausen neurofibromatosis. Genes Chrom. Cancer 3, 62–70 (1991).
Xu, W. et al. Loss of alleles in pheochromocytomas from patients with type 1 neurofibromatosis. Genes Chrom. Cancer 4, 337–341 (1992).
Jacks, T. et al. Tumorigenic and developmental consequences of a targeted Nf1 mutation in the mouse. Nature Genet. 7, 353–361 (1994).
Bourne, H.R., Sanders, D.A. & McCormick, F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature 348, 125–132 (1990).
Bourne, H.R., Sanders, D.A. & McCormick, F. The GTPase superfamily: conserved structure and molecular mechanism. Nature 349, 117–127 (1991).
Hall, A. Signal transduction through small GTPases — a tale of two GAPs. Cell 69, 389–391 (1992).
Boguski, M. & McCormick, F. Proteins regulating Ras and its relatives. Nature 366, 643–653 (1993).
Basu, T.N. et al. Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature 356, 713–715 (1992).
DeClue, J.E. et al. Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell 69, 265–273 (1992).
The, I. et al. Neurofibromatosis type 1 gene mutations in neuroblastoma. Nature Genet. 3, 62–66 (1993).
Andersen, L.B. et al. Mutations in the neurofibromatosis 1 gene in sporadic malignant melanoma cell lines. Nature Genet. 3, 118–121 (1993).
Johnson, M.R., Look, A.T., DeClue, J.E., Valentine, M.B. & Lowy, D.R. Inactivation of the NF1 gene in human melanoma and neuroblastoma cell lines without impaired regulation of GTP-Ras. Proc. Natl. Acad. Sci. USA 90, 5539–5545 (1993).
Rodenhuis, S. Ras and human tumors. Semin. Cancer Biol. 3, 241–247 (1992).
Noda, M., Ko, M. & Ogura, A. Sarcoma viruses carrying the ras oncogene activate differentiation-associated properties of a neuronal cell line. Nature 318, 73–75 (1985).
Bar-Sagi, D. & Feramisco, J.R. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell 42, 841–648 (1985).
Ridley, A.J., Paterson, H.F., Noble, M. & Land, H. ras-mediated cell cycle arrest is altered by nuclear oncogenes to induce Schwann cell transformation. EMBO J. 7, 1635–1645 (1988).
Shannon, K.M. et al. Loss of the normal NF1 allele from the bone marrow of children with type 1 neurofibromatosis and malignant myeloid disorders. New Engl. J.Med. 330, 597–601 (1994).
Kalra, R., Paderanga, D., Olson, K. & Shannon, K.M. Genetic analysis is consistent with the hypothesis that NF1 limits myeloid cell growth through p21ras. Blood 84, 3435–3439 (1994).
Pierce, J.H. & Aaronson, S.A. Myeloid cell transformation by ras-containing murine sarcoma viruses. Mol. Cell. Biol. 5, 667–674 (1985).
Kahn, P. et al. v-erbA cooperates with sarcoma oncogenes in leukemic cell transformation. Cell 45, 349–356 (1986).
Bollag, G. & McCormick, F. Differential regulation of rasGAP and neurofibromatosis gene product activities. Nature 351, 576–579 (1991).
Emanuel, P.D., Bates, L.J., Castleberry, R.P., Gualtieri, R.J. & Zuckerman, K.S. Seletive hypersensitivity to granulocyte-macrophage colony stimulating factor by juvenile chronic myeloid leukemia hematopoietic progenitors. Blood 77, 925–929 (1991).
Freedman, M.H. et al. Central role of tumor necrosis factor, GM-CSF, and interieukin 1 in the pathogenesis of juvenile chronic myelogenous leukemia. Br. J. Haemal. 80, 40–48 (1992).
Brannan, C. et al. Targeted disruption of the neurofibromatosis, type 1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes Dev. 8, 1019–1029 (1994).
Johnson, M.R. et al. Neurofibromin can inhibit Ras-dependent growth by a mechanism independent of its GTPase-accelerating function. Mol. Cell. Biol. 14, 641–645 (1994).
Marshall, C.J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80, 179–185 (1995).
Neubauer, A., Shannon, K.M. & Liu, E. Mutations of the ras proto-oncogenes in childhood monosomy 7. Blood 77, 594–598 (1991).
Miyauchi, J. et al. Mutations of the N-ras gene in juvenile chronic myelogenous leukemia. Blood 83, 2248–2254 (1994).
Burgering, B.M., de Vries-Smits, A.M., Medema, R.H., van Weeren, P.C., Tertoolen, L.G. & Bos, J.L. Epidermal growth factor induces phosphorylation of extracellular signal-regulated kinase 2 via multiple pathways. Mol. Cell Biol. 13, 7248–7256 (1993).
Lanfrancone, L. et al. Overexpression of She proteins potentiates the proliferative response to the granulocyte-macrophage colony-stimulating factor and recruitment of Grb2/SOS and Grb2/p140 complexes to the β receptor subunit. Oncogene 10, 907–917 (1995).
Satoh, T. . et al. Accumulation of p21ras·GTP in response to stimulation with epidermal growth factor and oncogene products with tyrosine kinase activity. Proc. Natl. Acad. Sci. USA 87, 7926–7929 (1990).
Mui, A.L.-F., Miyajima, A. lnterleukin-3 and granulocyte-macrophage colony-stimulating factor receptor signal transduction. Proc. Soc. Exp. Biol. Med. 206, 284–288 (1994).
Johnson, G.R., Gonda, T.J., Metcalf, D., Hariharan, K. & Cory, S. A lethal myeloproliferative syndrome in mice transplanted with bone marrow cells infected with a retrovirus expressing granulocyte-macrophage colony stimulating factor. EMBO J. 8, 441–448 (1989).
Lang, R. et al. Transgenic mice expressing a hematopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell 51, 675–686 (1987).
Kaneko, Y. et al. Chromosome patterns in juvenile chronic myelogenous leukemia, myelodysplastic syndrome, and acute leukemia associated with neurofibromatosis. Leukemia 3, 36–41 (1989).
Vogel, K.S., Brannan, C.I., Jenkins, N.A., Copeland, N.G. & Parada, L.F. Loss of neurofibromin results in neurotrophin-independent survival of embryonic sensory and sympathetic neurons. Cell 82, 733–742 (1995).
Gibbs, J.B., Oliff, A. & Kohl, N.E. Farnesyltransferase inhibitors: Ras research yields a potential cancer therapeutic. Cell 77, 175–178 (1994).
Downward, J., Graves, J.D., Warne, P.H., Rayter, S. & Cantrell, D.A. Stimulation of p21ras upon T-cell activation. Nature 346, 719–723 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bollag, G., Clapp, D., Shih, S. et al. Loss of NF1 results in activation of the Ras signaling pathway and leads to aberrant growth in haematopoietic cells. Nat Genet 12, 144–148 (1996). https://doi.org/10.1038/ng0296-144
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng0296-144
This article is cited by
-
The role of CRAF in cancer progression: from molecular mechanisms to precision therapies
Nature Reviews Cancer (2024)
-
Analysis of clinical and genomic profiles of therapy-related myeloid neoplasm in Korea
Human Genomics (2023)
-
Neurobehavioral sex-related differences in Nf1+/− mice: female show a “camouflaging”-type behavior
Biology of Sex Differences (2023)
-
Epigenetic regulation by ASXL1 in myeloid malignancies
International Journal of Hematology (2023)
-
Breast MRI: Clinical Indications, Recommendations, and Future Applications in Breast Cancer Diagnosis
Current Oncology Reports (2023)