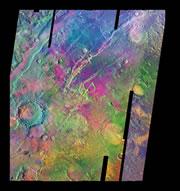
 This false colour image of Mars shows mounds of olivine minerals in purple, which could make methane.© NASA/JPL/ASU
This false colour image of Mars shows mounds of olivine minerals in purple, which could make methane.© NASA/JPL/ASUThe methane in Mars's atmosphere could easily be produced by mineral chemistry, rather than life. That's the claim from a pair of geologists whose calculations suggest that some experts have been too quick to assume a bacterial source for the gas.
When methane was found in the red planet's atmosphere last year1, scientists immediately realized that there must be a continuous or recent supply somewhere, because the average martian methane molecule is destroyed by sunlight after spending 340 years in the atmosphere (see 'Methane found on Mars').
Could this gas be a whiff of life from methane-producing bacteria, scientists wondered. One team calculated that it would take just 20 tonnes of bacteria to generate the observed concentration of methane in the atmosphere2.
But many geologists were sceptical, pointing out that minerals such as olivine can create methane in a process known as serpentinization. "Everyone says it could be serpentinization, but nobody had actually worked out how much olivine it would take," says Chris Oze, a geologist from Dartmouth College, Hanover, New Hampshire. "So we tried to provide a number."
He and his Dartmouth colleague Mukul Sharma calculated that the process would consume about 80,000 tonnes of olivine each year. To spit out methane at the same rate over the planet's 4.5-billion-year lifetime would require a global, 50-centimetre-thick layer of the mineral, spread a few kilometres below the planet's crust. That would be just one millionth of the mass of the planet. "It really doesn't take much olivine at all," says Oze.
Oze says that the calculation, published online in Geophysical Research Letters3, could swing the debate. "I'd love to see bugs," he laughs, "but you can't just go on hope. You have to consider the geological options."
Under pressure
When olivine is heated under pressure, it reacts with water and carbon dioxide to create methane, leaving the mineral serpentine behind. Geologists have calculated that the necessary conditions exist a few kilometres below Mars's surface. And we know that the red planet hosts some green olivine: the mineral has been found in martian meteorites, and has been spotted by both the Mars rover Opportunity and NASA's orbiting probe, Mars Global Surveyor4.
In a paper published this month in Geology5, the extent of one particular olivine field has been revised upwards by four times after an extensive analysis of data from NASA's Mars Odyssey orbiter. It is now thought to be the size of Cuba. The rock looks as though it was forced to the surface during ancient volcanic activity, so even more olivine may lie beneath the surface.
ADVERTISEMENT
"I think there's a lot of olivine on Mars," says Phil Christensen, the geologist from Arizona State University, Tempe, who analysed Odyssey's olivine findings. A global 50-centimetre layer "is plenty doable", he adds.
A crucial test of the serpentinization theory is whether the Mars rovers find serpentine, says Oze. On the other hand, the measurement of carbon isotopes in the methane, slated for a Mars mission at the end of the decade, might prove that bacteria are the source.
In the meantime, some scientists continue to debate whether there actually is any methane on the planet at all, and are holding out for further measurements.
-
References
- Formisano V., Atreya S., Encrenaz T., Ignatiev N. & Giuranna M. Science, 306. 1758 - 1761 (2004). | Article | PubMed | ChemPort |
- Krasnopolsky V. A., Maillard J. P. & Owen T. C. Icarus, 172. 537 - 547 (2004). | Article | ChemPort |
- Oze C. & Sharma M. Geophys. Res. Lett., published online doi10.1029/2005GL022691 (2005). | Article |
- Hoefen T. M. Science, 302. 627 - 630 (2003). | Article | PubMed | ISI | ChemPort |
- Hamilton V. E. Christensen P. R. Geology, 33. 433 - 436 (2005). | Article |
