Abstract
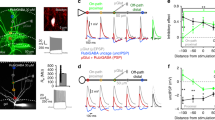
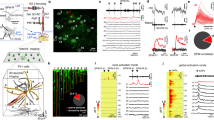
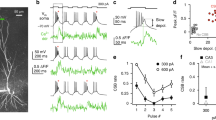
The perforant-path projection to the hippocampus forms synapses in the apical tuft of CA1 pyramidal neurons. We used computer modeling to examine the function of these distal synaptic inputs, which led to three predictions that we confirmed in experiments using rat hippocampal slices. First, activation of CA1 neurons by the perforant path is limited, a result of the long distance between these inputs and the soma. Second, activation of CA1 neurons by the perforant path depends on the generation of dendritic spikes. Third, the forward propagation of these spikes is unreliable, but can be facilitated by modest activation of Schaffer-collateral synapses in the upper apical dendrites. This 'gating' of dendritic spike propagation may be an important activation mode of CA1 pyramidal neurons, and its modulation by neurotransmitters or long-term, activity-dependent plasticity may be an important feature of dendritic integration during mnemonic processing in the hippocampus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Stuart, G., Spruston, N., Sakmann, B. & Häusser, M. Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci. 20, 125–131 (1997).
Johnston, D., Magee, J.C., Colbert, C.M. & Christie, B.R. Active properties of neuronal dendrites. Annu. Rev. Neurosci. 19, 165–186 (1996).
Häusser, M., Spruston, N. & Stuart, G.J. Diversity and dynamics of dendritic signaling. Science 290, 739–744 (2000).
Amaral, D.G. Emerging principles of intrinsic hippocampal organization. Curr. Opin. Neurobiol. 3, 225–229 (1993).
Colbert, C.M. & Levy, W.B. Electrophysiological and pharmacological characterization of perforant path synapses in CA1: mediation by glutamate receptors. J. Neurophysiol. 68, 1–8 (1992).
Desmond, N.L., Scott, C.A., Jane, J.A., Jr. & Levy, W.B. Ultrastructural identification of entorhinal cortical synapses in CA1 stratum lacunosum-moleculare of the rat. Hippocampus 4, 594–600 (1994).
Levy, W.B., Colbert, C.M. & Desmond, N.L. Another network model bites the dust: entorhinal inputs are no more than weakly excitatory in the hippocampal CA1 region. Hippocampus 5, 137–140 (1995).
Soltesz, I. Brief history of cortico-hippocampal time with a special reference to the direct entorhinal input to CA1. Hippocampus 5, 120–124 (1995).
Yeckel, M.F. & Berger, T.W. Monosynaptic excitation of hippocampal CA1 pyramidal cells by afferents from the entorhinal cortex. Hippocampus 5, 108–114 (1995).
Golding, N.L. & Spruston, N. Dendritic sodium spikes are variable triggers of axonal action potentials in hippocampal CA1 pyramidal neurons. Neuron 21, 1189–1200 (1998).
Kamondi, A., Acsady, L. & Buzsaki, G. Dendritic spikes are enhanced by cooperative network activity in the intact hippocampus. J. Neurosci. 18, 3919–3928 (1998).
Colbert, C.M. & Johnston, D. Axonal action-potential initiation and Na+ channel densities in the soma and axon initial segment of subicular pyramidal neurons. J. Neurosci. 16, 6676–6686 (1996).
Spruston, N., Schiller, Y., Stuart, G. & Sakmann, B. Activity-dependent action potential invasion and calcium influx into hippocampal CA1 dendrites. Science 268, 297–300 (1995).
Golding, N.L., Kath, W.L. & Spruston, N. Dichotomy of action-potential backpropagation in CA1 pyramidal neuron dendrites. J. Neurophysiol. 86, 2998–3010 (2001).
Hoffman, D.A., Magee, J.C., Colbert, C.M. & Johnston, D.K. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature 387, 869–875 (1997).
Migliore, M., Hoffman, D.A., Magee, J.C. & Johnston, D. Role of an A-type K+ conductance in the back-propagation of action potentials in the dendrites of hippocampal pyramidal neurons. J. Comput. Neurosci. 7, 5–15 (1999).
Poirazi, P., Brannon, T. & Mel, B.W. Arithmetic of subthreshold synaptic summation in a model CA1 pyramidal cell. Neuron 37, 977–987 (2003).
Golding, N.L., Staff, N.P. & Spruston, N. Dendritic spikes as a mechanism for cooperative long-term potentiation. Nature 418, 326–331 (2002).
Goldstein, S.S. & Rall, W. Changes of action potential shape and velocity for changing core conductor geometry. Biophys. J. 14, 731–757 (1974).
Manor, Y., Koch, C. & Segev, I. Effect of geometrical irregularities on propagation delay in axonal trees. Biophys. J. 60, 1424–1437 (1991).
Stuart, G.J. & Hausser, M. Dendritic coincidence detection of EPSPs and action potentials. Nat. Neurosci. 4, 63–71 (2001).
Anderson, P., Storm, J. & Wheal, H.V. Thresholds of action potentials evoked by synapses on the dendrites of pyramidal cells in the rat hippocampus in vitro. J. Physiol. (Lond.) 383, 509–526 (1987).
Andersen, P. & Lomo, T. Mode of activation of hippocampal pyramidal cells by excitatory synapses on dendrites. Exp. Brain Res. 2, 247–260 (1966).
Buzsaki, G., Penttonen, M., Bragin, A., Nadasdy, Z. & Chrobak, J.J. Possible physiological role of the perforant path-CA1 projection. Hippocampus 5, 141–146 (1995).
Empson, R.M. & Heinemann, U. The perforant path projection to hippocampal area CA1 in the rat hippocampal-entorhinal cortex combined slice. J. Physiol. (Lond.) 484, 707–720 (1995).
Empson, R.M. & Heinemann, U. Perforant path connections to area CA1 are predominantly inhibitory in the rat hippocampal-entorhinal cortex combined slice preparation. Hippocampus 5, 104–107 (1995).
Remondes, M. & Schuman, E.M. Direct cortical input modulates plasticity and spiking in CA1 pyramidal neurons. Nature 416, 736–740 (2002).
Pare, D. & Llinas, R. Intracellular study of direct entorhinal inputs to field CA1 in the isolated guinea pig brain in vitro. Hippocampus 5, 115–119 (1995).
Doller, H.J. & Weight, F.F. Perforant pathway activation of hippocampal CA1 stratum pyramidale neurons: electrophysiological evidence for a direct pathway. Brain Res. 237, 1–13 (1982).
Yeckel, M.F. & Berger, T.W. Feedforward excitation of the hippocampus by afferents from the entorhinal cortex: redefinition of the role of the trisynaptic pathway. Proc. Natl. Acad. Sci. USA 87, 5832–5836 (1990).
Brun, V.H. et al. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science 296, 2243–2246 (2002).
McNaughton, B.L., Barnes, C.A., Meltzer, J. & Sutherland, R.J. Hippocampal granule cells are necessary for normal spatial learning but not for spatially-selective pyramidal cell discharge. Exp. Brain Res. 76, 485–496 (1989).
Gasparini, S., Migliore, M. & Magee, J.C. On the initiation and propagation of dendritic spikes in CA1 pyramidal neurons. J. Neurosci. 24, 11046–11056 (2004).
Fellous, J.M., Rudolph, M., Destexhe, A. & Sejnowski, T.J. Synaptic background noise controls the input/output characteristics of single cells in an in vitro model of in vivo activity. Neuroscience 122, 811–829 (2003).
Frick, A., Magee, J. & Johnston, D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat. Neurosci. 7, 126–135 (2004).
Johnston, D., Hoffman, D.A., Colbert, C.M. & Magee, J.C. Regulation of back-propagating action potentials in hippocampal neurons. Curr. Opin. Neurobiol. 9, 288–292 (1999).
Spencer, W.A. & Kandel, E.R. Electrophysiology of hippocampal neurons. IV. Fast prepotentials. J. Neurophysiol. 24, 272–285 (1961).
Wong, R.K. & Stewart, M. Different firing patterns generated in dendrites and somata of CA1 pyramidal neurones in guinea-pig hippocampus. J. Physiol. (Lond.) 457, 675–687 (1992).
Cauller, L.J. & Connors, B.W. Functions of very distal dendrites: experimental and computational studies of layer I inputs to layer V pyramidal neurons in neocortex. in Single Neuron Computation (eds. McKenna, T., Davis, J. & Zornetzer, S.F.) 199–230 (Academic Press, San Diego, 1992).
Larkum, M.E., Senn, W. & Luscher, H.R. Top-down dendritic input increases the gain of layer 5 pyramidal neurons. Cereb. Cortex 14, 1059–1070 (2004).
Larkum, M.E., Zhu, J.J. & Sakmann, B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature 398, 338–341 (1999).
Hines, M.L. & Carnevale, N.T. The NEURON simulation environment. Neural Comput. 9, 1179–1209 (1997).
Megias, M., Emri, Z., Freund, T.F. & Gulyas, A.I. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience 102, 527–540 (2001).
Magee, J.C. & Cook, E.P. Somatic EPSP amplitude is independent of synapse location in hippocampal pyramidal neurons. Nat. Neurosci. 3, 895–903 (2000).
Acknowledgements
We thank members of the Spruston and Kath labs and B. Mel for discussions. This work was supported by the US National Institutes of Health (NS-35180 to N.S., NS-46064 to N.S. and W.L.K., and NS-045437 to T.J.) and by the National Science Foundation (DGE-9987577 to A.R.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Successful forward propagation of dendritic spikes in strong dendritic excitability models (model cells 1 and 2, with animations). (PDF 709 kb)
Supplementary Fig. 2
Gating of PP-evoked dendritic spikes by SC EPSPs (model cell 1, with animations). (PDF 846 kb)
Supplementary Fig.3
Gating of PP-evoked dendritic spikes by SC EPSPs (model cell 2, with animations). (PDF 1246 kb)
Supplementary Fig. 4
Gating of PP-evoked dendritic spikes by SC EPSPs (model cell 3). (PDF 646 kb)
Supplementary Fig. 5
Bidirectional gating of dendritic spike propagation by excitation and feed-forward inhibition. (PDF 241 kb)
Rights and permissions
About this article
Cite this article
Jarsky, T., Roxin, A., Kath, W. et al. Conditional dendritic spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons. Nat Neurosci 8, 1667–1676 (2005). https://doi.org/10.1038/nn1599
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1599
This article is cited by
-
A voltage-based Event-Timing-Dependent Plasticity rule accounts for LTP subthreshold and suprathreshold for dendritic spikes in CA1 pyramidal neurons
Journal of Computational Neuroscience (2024)
-
Heterosynaptic plasticity-induced modulation of synapses
The Journal of Physiological Sciences (2023)
-
Introducing the Dendrify framework for incorporating dendrites to spiking neural networks
Nature Communications (2023)
-
Dendritic excitability controls overdispersion
Nature Computational Science (2023)
-
Sharp-wave ripple doublets induce complex dendritic spikes in parvalbumin interneurons in vivo
Nature Communications (2022)