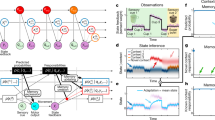
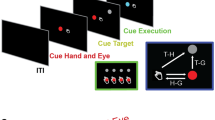
Abstract
Behaviors such as sensing an object and then moving your eyes or your hand toward it require that sensory information be used to help generate a motor command, a process known as a sensorimotor transformation. Here we review models of sensorimotor transformations that use a flexible intermediate representation that relies on basis functions. The use of basis functions as an intermediate is borrowed from the theory of nonlinear function approximation. We show that this approach provides a unifying insight into the neural basis of three crucial aspects of sensorimotor transformations, namely, computation, learning and short-term memory. This mathematical formalism is consistent with the responses of cortical neurons and provides a fresh perspective on the issue of frames of reference in spatial representations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Tresch, M., Saltiel, P. & Bizzi, E. The construction of movement by the spinal cord. Nat. Neurosci. 2, 162–167 (1999).
Todorov, E. Direct cortical control of muscle activation in voluntary arm movements: a model. Nat. Neurosci. 3, 391– 398 (2000).
Poggio, T. A theory of how the brain might work. Cold Spring Harbor Symp. Quant. Biol. 55, 899–910 (1990).
Pouget, A. & Sejnowski, T. A neural model of the cortical representation of egocentric distance. Cereb. Cortex 4, 314–329 (1994).
Pouget, A. & Sejnowski, T. Spatial transformations in the parietal cortex using basis functions. J. Cogn. Neurosci. 9, 222–237 (1997).
Groh, J. & Sparks, D. Two models for transforming auditory signals from head-centered to eye-centered coordinates. Biol. Cybern. 67, 291–302 ( 1992).
Burnod, Y. et al. Visuomotor transformations underlying arm movements toward visual targets: a neural network model of cerebral cortical operations. J. Neurosci. 12, 1435–1453 (1992).
Salinas, E. & Abbot, L. Transfer of coded information from sensory to motor networks. J. Neurosci. 15, 6461–6474 (1995).
Zipser, D. & Andersen, R. A back-propagation programmed network that stimulates reponse properties of a subset of posterior parietal neurons . Nature 331, 679–684 (1988).
Andersen, R., Essick, G. & Siegel, R. Encoding of spatial location by posterior parietal neurons . Science 230, 456–458 (1985).
Trotter, Y., Celebrini, S., Stricanne, B., Thorpe, S. & Imbert, M. Neural processing of stereopsis as a function of viewing distance in primate visual area V1. J. Neurophysiol. 76, 2872–2885 ( 1997).
Trotter, Y. & Celebrini, S. Gaze direction controls response gain in primary visual-cortex neurons. Nature 398, 239–242 (1999).
Galletti, C. & Battaglini, P. Gaze-dependent visual neurons in area {V3a} of monkey prestriate cortex. J. Neurosci. 9, 1112–1125 (1989).
Bremmer, F., Ilg, U., Thiele, A., Distler, C. & Hoffman, K. Eye position effects in monkey cortex. I: Visual and pursuit-related activity in extrastriate areas MT and MST. J. Neurophysiol. 77, 944–961 ( 1997).
Cumming, B. & Parker, A. Binocular neurons in V1 of awake monkeys are selective for absolute, not relative, disparity. J. Neurosci. 19, 5602–5618 (1999).
Boussaoud, D., Barth, T. & Wise, S. Effects of gaze on apparent visual responses of frontal cortex neurons. Exp. Brain Res. 93, 423–434 (1993).
Squatrito, S. & Maioli, M. Gaze field properties of eye position neurones in areas MST and 7a of macaque monkey. Vis. Neurosci. 13, 385–398 ( 1996).
Vallar, G. Spatial hemineglect in humans. Trends Cogn. Sci. 2, 87–97 (1998).
Pouget, A., Deneve, S. & Sejnowski, T. Frames of reference in hemineglect: a computational approach. Prog. Brain Res. 121, 81– 97 (1999).
Piaget, J. The Origins of Intelligence in Children (The Norton Library, New York, 1952).
Kuperstein, M. Neural model of adaptative hand-eye coordination for single postures. Science 239, 1308–1311 ( 1988).
Widrow, B. & Hoff, M. E. in Conference proceedings of WESCON , 96–104 (1960).
Moody, J. & Darken, C. Fast learning in networks of locally-tuned processing units. Neural Comput. 1, 281– 294 (1989).
Hinton, G. & Brown, A. in Neural Information Processing Systems vol. 12, 122–128 (MIT Press, Cambridge Massachusetts, 2000).
Olshausen, B. A. & Field, D. J. Sparse coding with an overcomplete basis set: a strategy employed by V1? Vision Res. 37, 3311–3325 ( 1997).
Jordan, M. & Rumelhart, D. Forward models: supervised learning with a distal teacher. Cognit. Sci. 16, 307–354 (1990).
Rumelhart, D., Hinton, G. & Williams, R. in Parallel Distributed Processing (eds. Rumelhart, D., McClelland, J. & Group, P. R.) 318–362 (MIT Press, Cambridge, Massachusetts, 1986).
Desmurget, M. et al. Role of the posterior parietal cortex in updating reaching movements to a visual target. Nat. Neurosci. 2, 563–567 (1999).
Wolpert, D. M., Goodbody, S. J. & Husain, M. Maintaining internal representations: the role of the human superior parietal lobe. Nat. Neurosci. 1, 529–533 (1998).
Amit, D. The hebbian paradigm reintegrated — local reverberations as internal representations. Behav. Brain Sci. 18, 617 –626 (1995).
Fuster, J. Memory in the Cerebral Cortex: An Empirical Approach to Neural Networks in the Human and Nonhuman Primate (MIT Press, Cambridge, Massachusetts, 1995).
Goldberg, M. & Bruce, C. Primate frontal eye fields. III. Maintenance of a spatially accurate saccade signal. J. Neurophysiol. 64, 489–508 (1990).
Gnadt, J. & Mays, L. Neurons in monkey parietal area LIP are tuned for eye-movement parameters in three-dimensional space. J. Neurophysiol. 73, 280–297 (1995).
Funahashi, S., Bruce, C. & Goldman-Rakic, P. Dorsolateral prefrontal lesions and oculomotor delayed response performance: evidence for mnemonic “scotomas”. J. Neurosci. 13, 1479–1497 (1993).
Zhang, K. Representation of spatial orientation by the intrinsic dynamics of the head-direction cell ensemble: a theory. J. Neurosci. 16, 2112–2126 (1996).
Somers, D. C., Nelson, S. B. & Sur, M. An emergent model of orientation selectivity in cat visual cortical simple cells. J. Neurosci. 15, 5448–5465 (1995).
Salinas, E. & Abbott, L. F. A model of multiplicative neural responses in parietal cortex. Proc. Natl. Acad. Sci. USA 93, 11956–11961 (1996).
Deneve, S., Latham, P. & Pouget, A. Reading population codes: A neural implementation of ideal observers. Nat. Neurosci. 2, 740– 745 (1999).
Walker, M., Fitzgibbon, E. & Goldberg, M. Neurons in the monkey superior colliculus predict the visual result of impending saccadic eye movements. J. Neurophysiol. 73, 1988–2003 ( 1995).
Mazzoni, P., Bracewell, R., Barash, S. & Andersen, R. Motor intention activity in the macaque's lateral intraparietal area. I. Dissociation of motor plan from sensory memory. J. Neurophysiol. 76, 1439–1456 (1996).
Graziano, M., Hu, X. & Gross, C. Coding the locations of objects in the dark. Science 277, 239–241 (1997).
Duhamel, J. R., Colby, C. L. & Goldberg, M. E. The updating of the representation of visual space in parietal cortex by intended eye movements. Science 255, 90–92 (1992).
Droulez, J. & Berthoz, A. A neural model of sensoritopic maps with predictive short-term memory properties. Proc. Natl. Acad. Sci. USA 88, 9653–9657 ( 1991).
Dominey, P. & Arbib, M. A cortico-subcortical model for the generation of spatially accurate sequential saccades. Cereb. Cortex 2, 153–175 ( 1992).
Seung, H. How the brain keeps the eyes still. Proc. Natl. Acad. Sci. USA 93, 13339–13344 ( 1996).
Snyder, L., Batista, A. & Andersen, R. Coding of intention in the posterior parietal cortex . Nature 386, 167–170 (1997).
Snyder, L., Grieve, K., Brotchie, P. & Andersen, R. Separate body- and world-referenced representations of visual space in parietal cortex. Nature 394, 887–891 ( 1998).
Acknowledgements
A.P. is supported by a Young Investigator Award from ONR and fellowships from the Sloan Foundation and the McDonnell-Pew foundation. L.H.S. is supported by fellowships from the Sloan and Klingenstein foundations, and by NEI. We thank Daphne Bavelier and Suliann Ben Hamed for comments on earlier versions of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pouget, A., Snyder, L. Computational approaches to sensorimotor transformations. Nat Neurosci 3 (Suppl 11), 1192–1198 (2000). https://doi.org/10.1038/81469
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/81469
This article is cited by
-
Cerebro-cerebellar networks facilitate learning through feedback decoupling
Nature Communications (2023)
-
Deep Intelligence: What AI Should Learn from Nature’s Imagination
Cognitive Computation (2023)
-
Eye tracking to assess concussions: an intra-rater reliability study with healthy youth and adult athletes of selected contact and collision team sports
Experimental Brain Research (2021)
-
Spiking neurons with spatiotemporal dynamics and gain modulation for monolithically integrated memristive neural networks
Nature Communications (2020)
-
Mechanisms underlying gain modulation in the cortex
Nature Reviews Neuroscience (2020)