Abstract
Aberrant epidermal growth factor receptor (EGFR) signaling is widespread in cancer, making the EGFR an important target for therapy. EGFR gene amplification and mutation are common in glioblastoma (GBM), but EGFR inhibition has not been effective in treating this tumor. Here we propose that primary resistance to EGFR inhibition in glioma cells results from a rapid compensatory response to EGFR inhibition that mediates cell survival. We show that in glioma cells expressing either EGFR wild type or the mutant EGFRvIII, EGFR inhibition triggers a rapid adaptive response driven by increased tumor necrosis factor (TNF) secretion, which leads to activation in turn of c-Jun N-terminal kinase (JNK), the Axl receptor tyrosine kinase and extracellular signal–regulated kinases (ERK). Inhibition of this adaptive axis at multiple nodes rendered glioma cells with primary resistance sensitive to EGFR inhibition. Our findings provide a possible explanation for the failures of anti-EGFR therapy in GBM and suggest a new approach to the treatment of EGFR-expressing GBM using a combination of EGFR and TNF inhibition.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sharma, S.V., Bell, D.W., Settleman, J. & Haber, D.A. Epidermal growth factor receptor mutations in lung cancer. Nat. Rev. Cancer 7, 169–181 (2007).
Arteaga, C.L. & Engelman, J.A. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 25, 282–303 (2014).
Chong, C.R. & Jänne, P.A. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat. Med. 19, 1389–1400 (2013).
Fan, W. et al. MET-independent lung cancer cells evading EGFR kinase inhibitors are therapeutically susceptible to BH3 mimetic agents. Cancer Res. 71, 4494–4505 (2011).
Lee, H.J. et al. Drug resistance via feedback activation of Stat3 in oncogene-addicted cancer cells. Cancer Cell 26, 207–221 (2014).
Verhaak, R.G. et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17, 98–110 (2010).
Hatanpaa, K.J., Burma, S., Zhao, D. & Habib, A.A. Epidermal growth factor receptor in glioma: signal transduction, neuropathology, imaging, and radioresistance. Neoplasia 12, 675–684 (2010).
Mellinghoff, I.K. et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med. 353, 2012–2024 (2005).
Karpel-Massler, G., Schmidt, U., Unterberg, A. & Halatsch, M.E. Therapeutic inhibition of the epidermal growth factor receptor in high-grade gliomas: where do we stand? Mol. Cancer Res. 7, 1000–1012 (2009).
Akhavan, D. et al. De-repression of PDGFRβ transcription promotes acquired resistance to EGFR tyrosine kinase inhibitors in glioblastoma patients. Cancer Discov. 3, 534–547 (2013).
Nathanson, D.A. et al. Targeted therapy resistance mediated by dynamic regulation of extrachromosomal mutant EGFR DNA. Science 343, 72–76 (2014).
Barkovich, K.J. et al. Kinetics of inhibitor cycling underlie therapeutic disparities between EGFR-driven lung and brain cancers. Cancer Discov. 2, 450–457 (2012).
Wykosky, J. et al. A urokinase receptor-Bim signaling axis emerges during EGFR inhibitor resistance in mutant EGFR glioblastoma. Cancer Res. 75, 394–404 (2015).
Chakraborty, S. et al. Constitutive and ligand-induced EGFR signalling triggers distinct and mutually exclusive downstream signalling networks. Nat. Commun. 5, 5811 (2014).
Guo, G. et al. Ligand-independent EGFR signaling. Cancer Res. 75, 3436–3441 (2015).
Acquaviva, J. et al. Chronic activation of wild-type epidermal growth factor receptor and loss of Cdkn2a cause mouse glioblastoma formation. Cancer Res. 71, 7198–7206 (2011).
Endres, N.F. et al. Conformational coupling across the plasma membrane in activation of the EGF receptor. Cell 152, 543–556 (2013).
Ramnarain, D.B. et al. Differential gene expression analysis reveals generation of an autocrine loop by a mutant epidermal growth factor receptor in glioma cells. Cancer Res. 66, 867–874 (2006).
Wong, A.J. et al. Structural alterations of the epidermal growth factor receptor gene in human gliomas. Proc. Natl. Acad. Sci. USA 89, 2965–2969 (1992).
Gil Del Alcazar, C.R., Todorova, P.K., Habib, A.A., Mukherjee, B. & Burma, S. Augmented HR repair mediates acquired temozolomide resistance in glioblastoma. Mol. Cancer Res. 14, 928–940 (2016).
Pingle, S.C. et al. In silico modeling predicts drug sensitivity of patient-derived cancer cells. J. Transl. Med. 12, 128 (2014).
Puliyappadamba, V.T. et al. Opposing effect of EGFRWT on EGFRvIII-mediated NF-κB activation with RIP1 as a cell death switch. Cell Rep. 4, 764–775 (2013).
Sarkaria, J.N. et al. Use of an orthotopic xenograft model for assessing the effect of epidermal growth factor receptor amplification on glioblastoma radiation response. Clin. Cancer Res. 12, 2264–2271 (2006).
Stommel, J.M. et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science 318, 287–290 (2007).
Huang, P.H. et al. Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc. Natl. Acad. Sci. USA 104, 12867–12872 (2007).
Pillay, V. et al. The plasticity of oncogene addiction: implications for targeted therapies directed to receptor tyrosine kinases. Neoplasia 11, 448–458 (2009).
Greenall, S.A. et al. EGFRvIII-mediated transactivation of receptor tyrosine kinases in glioma: mechanism and therapeutic implications. Oncogene 34, 5277–5287 (2015).
Awad, A.J., Burns, T.C., Zhang, Y. & Abounader, R. Targeting MET for glioma therapy. Neurosurg. Focus 37, E10 (2014).
Zhu, Y. & Shah, K. Multiple lesions in receptor tyrosine kinase pathway determine glioblastoma response to pan-ERBB inhibitor PF-00299804 and PI3K/mTOR dual inhibitor PF-05212384. Cancer Biol. Ther. 15, 815–822 (2014).
Blakely, C.M. et al. NF-κB-activating complex engaged in response to EGFR oncogene inhibition drives tumor cell survival and residual disease in lung cancer. Cell Rep. 11, 98–110 (2015).
Wajant, H., Pfizenmaier, K. & Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ. 10, 45–65 (2003).
Higuchi, M. & Aggarwal, B.B. TNF induces internalization of the p60 receptor and shedding of the p80 receptor. J. Immunol. 152, 3550–3558 (1994).
Deng, L., Ding, W. & Granstein, R.D. Thalidomide inhibits tumor necrosis factor-alpha production and antigen presentation by Langerhans cells. J. Invest. Dermatol. 121, 1060–1065 (2003).
Gross, N.D. et al. Inhibition of Jun NH2-terminal kinases suppresses the growth of experimental head and neck squamous cell carcinoma. Clin. Cancer Res. 13, 5910–5917 (2007).
Groves, M.D. et al. A North American Brain Tumor Consortium (NABTC 99-04) phase II trial of temozolomide plus thalidomide for recurrent glioblastoma multiforme. J. Neurooncol. 81, 271–277 (2007).
Gu, X. et al. Intraperitoneal injection of thalidomide attenuates bone cancer pain and decreases spinal tumor necrosis factor-α expression in a mouse model. Mol. Pain 6, 64 (2010).
Prahallad, A. et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 483, 100–103 (2012).
Duncan, J.S. et al. Dynamic reprogramming of the kinome in response to targeted MEK inhibition in triple-negative breast cancer. Cell 149, 307–321 (2012).
Huang, P.H., Xu, A.M. & White, F.M. Oncogenic EGFR signaling networks in glioma. Sci. Signal 2, re6 (2009).
Li, L. et al. An EGFR wild type-EGFRvIII-HB-EGF feed-forward loop regulates the activation of EGFRvIII. Oncogene 33, 4253–4264 (2014).
Fan, Q.W. et al. EGFR phosphorylates tumor-derived EGFRvIII driving STAT3/5 and progression in glioblastoma. Cancer Cell 24, 438–449 (2013).
Jahani-Asl, A. et al. Control of glioblastoma tumorigenesis by feed-forward cytokine signaling. Nat. Neurosci. 19, 798–806 (2016).
de la Iglesia, N. et al. Identification of a PTEN-regulated STAT3 brain tumor suppressor pathway. Genes Dev. 22, 449–462 (2008).
Rich, J.N. et al. Phase II trial of gefitinib in recurrent glioblastoma. J. Clin. Oncol. 22, 133–142 (2004).
Brown, P.D. et al. Phase I/II trial of erlotinib and temozolomide with radiation therapy in the treatment of newly diagnosed glioblastoma multiforme: North Central Cancer Treatment Group Study N0177. J. Clin. Oncol. 26, 5603–5609 (2008).
Peereboom, D.M. et al. Phase II trial of erlotinib with temozolomide and radiation in patients with newly diagnosed glioblastoma multiforme. J. Neurooncol. 98, 93–99 (2010).
Schuster, J. et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro-oncol. 17, 854–861 (2015).
Inda, M.D. et al. Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev. 24, 1731–1745 (2010).
Furnari, F.B., Cloughesy, T.F., Cavenee, W.K. & Mischel, P.S. Heterogeneity of epidermal growth factor receptor signalling networks in glioblastoma. Nat. Rev. Cancer 15, 302–310 (2015).
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005).
Acknowledgements
We thank F. Furnari (University of California, San Diego), C.D. James (Northwestern University) and E. Burstein (UT Southwestern Medical Center) for providing cell lines and reagents. This work was supported in part by NIH grants RO1 NS062080 and by the Office of Medical Research, Departments of Veterans Affairs to A.A.H. (I01BX002559). S.B. is supported by grants from the National Institutes of Health (RO1CA149461, RO1CA197796 and R21CA202403) and the National Aeronautics and Space Administration (NNX16AD78G). This work was also supported by NIH grant 1R01CA194578 to D.Z.
Author information
Authors and Affiliations
Contributions
A.A.H., G.G. and K.G. designed experiments. G.G., K.G., S.A., N.A., S.S. and D.H.W. performed or assisted with experiments. B.M., K.J.H., J.N.S. and S.K. provided tumor samples. E.P., S.B., D.Z., J.N.S., G.G. and A.A.H. analyzed data. A.A.H. and G.G. wrote the manuscript with contributions from K.G., E.P. and K.J.H. A.A.H. supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
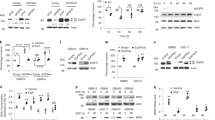
Supplementary Figure 1 EGFR inhibition activates signaling pathways
(a-b) EGFR inhibition activates signaling pathways (a) U251EGFR cells were treated with erlotinib (1μM) for the indicated times followed by Western blot with the indicated antibodies. (b) GBM9 neurospheres were treated with afatinib (100nM) for the indicated time points followed by Western blot with the indicated antibodies. (c-g) EGFR inhibition decreases pSTAT3 and pAKT activation. Glioma cells were treated with high dosage of erlotinib (10μM) or afatinib (10μM). Western blot showing that pSTAT3 and pAKT decrease upon treatment with erlotinib or afatinib. Western blots are representative of at least three independent replicates. (h-j) pERK is activated in U87EGFRwt and blocked by cetuximab. (h) U87EGFRwt cells were cultured in medium with (10%) or without serum in DMEM. pERK is activated under both conditions. (i) Cells were serum starved and treated overnight with cetuximab (100 μg/ml). Cetuximab treatment blocks both EGFR and ERK activation. (j) Cells were treated wtith EGF (50 ng/ml) for 15 min. EGF increases pERK level in U87EGFRwt cells. SF:Serum free. Western blots are representative of at least three independent replicates. Full-length blots are presented in Supplementary Figure 15.
Supplementary Figure 2 Intracellular signaling networks and biological responses to EGFR inhibition in glioma cells.
(a-b) EGFR inhibition does not activate NF-κB transcriptional activity. (a) A luciferase reporter assay shows that EGFR inhibition with erlotinib does not result in an increase in NF-κB reporter activity in GBM9 neurospheres and U87EGFRwt cells. Erlotinib was used for 24h at a concentration of 100 nM for GBM9 neurospheres and 1uM for U87EGFRwt cells. GBM9: Ctrl vs erlotinib: P =0.88, t=0.16, d.f.=4; U87EGFRwt: Ctrl vs erlotinib: P =0.60, t=0.57, d.f.=4. (b) As a positive control we used LPS (1μg/ml) which activates the NF-κB reporter. Renilla luciferase was used as an internal control. Ctrl vs LPS: P =0.0012, t=8.27, d.f.=4. Data are presented as mean±s.e.m; **P < 0.01 from two-tailed unpaired Student's t-test (n = 3 biologically independent experimental replicates). n.s. not significant. (c-d) Upregulation of TNF in response to EGFR inhibition in multiple patient derived samples and established cell lines. (c) SK987, SK748. SK1422, and GBM622 are patient derived primary GBM cells cultured as neurospheres and treated with erlotinib (100nM) for 24h followed by extraction of RNA and qRT-PCR for TNF. SB19vIII and U251EGFRwt cells are established GBM cell lines that were treated with erlotinib (1μM) for 24h followed by qRT-PCR for TNF. SB19vIII: Ctrl vs Erlotinib: P =0.0014,t=7.88, d.f.=4; SK987: Ctrl vs erlotinib: P =0.0024,t=6.83, d.f.=4; SK748: Ctrl vs Erlotinib: P =0.0019, t=7.32, d.f.=4; SK1422: Ctrl vs erlotinib: P =0.0030,t=6.45, d.f.=4; GBM622: Ctrl vs erlotinib: P =0.21,t=1.5, d.f.=4; U251EGFRwt: Ctrl vs erlotinib: P =0.0005, t=10.4, d.f.=4. Data are presented as mean±s.e.m; **P < 0.01, ***P<0.001 from two-tailed unpaired Student's t-test (n = 3 biologically independent experimental replicates). n.s. not significant. (d) Western blot showing EGFR levels in various neurospheres and cell lines. No increase in TNF is detected in GBM622 cells expressing a very low level of EGFR. Western blots are representative of at least three independent replicates. Full-length blots are presented in Supplementary Figure 15. (e) Downregulation of TNFR1 in response to EGFR inhibition in patient derived primary GBM neurosphere cultures and established cell lines. GBM9, SK987 and U87EGFRwt cells were treated with erlotinib for the indicated time points followed by Western blot with TNFR1 antibodies. Erlotinib was used at a concentration of 100 nM for GBM9 and SK987 and 1uM for U87EGFRwt cells. Western blots are representative of at least three independent replicates. (f) EGFR inhibition induced effects on cell viability are influenced by specific inhibitors of downstream signaling pathways. In this experiment primary GBM9 neurospheres were exposed to various inhibitors alone or in combination with erlotinib (100 nM) for 72h followed by Alamarblue Cell Viability assay. The concentrations of the various drugs were: SB203850 (10uM), SP600125 (1uM), U0126 (1uM), BMS-345541 (10uM), XL765 (10uM), Necrostatin-1 (300nM). Erlotinib vs erlotinib +SP600125: P =0.0001,t=14.96, d.f.=4; Erlotinib vs erlotinib +U0126: P =0.0018, t=7.42, d.f.=4. Data are presented as mean±s.e.m; **P < 0.01, ***P<0.001 from two-tailed unpaired Student's t-test (n = 3 biologically independent experimental replicates). (g-i) Western blot showing efficient silencing of JNK1/2, Axl and TNFR1. For cell viability experiments involving siRNA knockdown of JNK1/2, Axl and TNFR1, we confirmed silencing by Western blot. Western blots are representative of at least three independent replicates. Full-length blots are presented in Supplementary Figure 15.
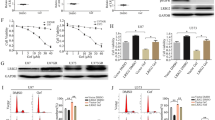
Supplementary Figure 3 EGFR inhibition induces a biologically significant upregulation of TNF in glioma cells.
(a-b) Thalidomide blocks EGFR inhibition induced upregulation of TNF. Patient derived GBM9 neurospheres or established GBM cell line U87EGFRwt cells were treated with erlotinib with or without thalidomide (1μM) followed by collection of supernatants and TNF ELISA. (a) Erlotinib vs erlotinib +thalidomide: P =0.0015, t=7.77, d.f.=4. (b) Erlotinib vs erlotinib +thalidomide: P =0.0008, t=8.98, d.f.=4. Data are presented as mean±s.e.m; **P < 0.01, ***P<0.001 from two-tailed unpaired Student's t-test (n = 3 biologically independent experimental replicates). (c-d) A neutralizing antibody to TNF sensitizes cells to EGFR inhibition. Patient derived GBM9 neurospheres or established GBM cell line U87EGFRwt cells were exposed to erlotinib plus either normal mouse IgG (Ctrl) or a neutralizing antibody to TNF. The TNF neutralizing antibody sensitizes cells to EGFR inhibition as shown by the Alamarblue Cell Viability assay. (c) Erlotinib vs erlotinib+TNF Ab: P =0.0013,t=8.07, d.f.=4. (d) Erlotinib vs erlotinib+TNF Ab: P =0.0012, t=8.13, d.f.=4. Data are presented as mean±s.e.m; **P < 0.01 from two-tailed unpaired Student's t-test (n =3 biologically independent experimental replicates). (e-f) Thalidomide sensitizes patient derived primary GBM9 and GBM39 neurospheres to EGFR inhibiton. GBM9 or GBM39 neurospheres were exposed to EGFR inhibition using afatinib (100nM) and thalidomide (1μM) alone or in combination concurrently for 72h followed by Alamarblue Cell Viability Assay. DMSO was used as a control (Ctrl). (e) Afatinib vs afatinib+thalidomide: P =0.0001,t=14.47, d.f.=4. (f) Afatinib vs afatinib+thalidomide: P =0.0005, t=10.4, d.f.=4. Data are presented as mean±s.e.m; ***P<0.001 from two-tailed unpaired Student's t-test (n =3 biologically independent experimental replicates).
Supplementary Figure 4 Inhibition of JNK and ERK sensitizes glioma cells to EGFR inhibition.
(a-b) GBM9 cells were treated with erlotinib and/or JNK inhibitor SP600125 (1μM), ERK inhibitor U0126 (1μM) for 72 hours followed by Annexin-FACS assay. Unstained cells represent viable cells. Annexin positive cells are undergoing apoptosis. PI (Propidium iodide) positive cells are undergoing late apoptosis. In “Control” cells are treated with control vehicle (DMSO). The combination treatment decreases the number of viable cells and there is an increase in double stained cells (Annexin+PI Positive). (b) Unstained: Erlotinib vs Erlotinib+SP600125: P =0.0008, t=9.10, d.f.=4; Erlotinib vs erlotinib+U0126: P =0.0036, t=6.14, d.f.=4. Annexin+PI: Erlotinib vs erlotinib+SP600125: P =0.0003, t=11.37, d.f.=4; Erlotinib vs erlotinib+U0126: P =0.0021,t=7.04, d.f.=4. (c-d) A similar experiment was conducted in U87EGFR cells with a similar result. (d) Unstained: Erlotinib vs erlotinib+SP600125: P =0.0045, t=5.77, d.f.=4; Erlotinib vs erlotinib+U0126: P =0.0013,t=8.06, d.f.=4. Annexin+PI: Erlotinib vs erlotinib+SP600125: P =0.0017, t=7.53, d.f.=4; Erlotinib vs erlotinib+U0126: P =0.0006, t=10.04, d.f.=4. Data are presented as mean±s.e.m; **P<0.01, ***P<0.001 using two-tailed unpaired Student's t-test (n =3 biologically independent experimental replicates).
Supplementary Figure 5 Axl and TNF inhibition renders glioma cells sensitive to EGFR inhibition.
(a-b) GBM9 cells were treated with erlotinib and/or Axl inhibitor R428 (1μM), Thalidomide (1μM) for 72 hours followed by Annexin-FACS assay. Unstained cells represent viable cells. Annexin positive cells are undergoing apoptosis. PI (Propidium iodide) positive cells are undergoing late apoptosis. In “Control” cells are treated with control vehicle (DMSO). The combination treatment decreases the number of viable cells and there is an increase in both PI and double stained cells (Annexin+PI Positive). (b) Unstained: Erlotinib vs erlotinib+R428: P =0.0051,t=5.58, d.f.=4; Erlotinib vs erlotinib+thalidomide: P =0.0009,t=8.75, d.f.=4. Annexin+PI: Erlotinib vs erlotinib+R428: P =0.015, t=4.10, d.f.=4; Erlotinib vs erlotinib+thalidomide: P =0.0011,t=8.32, d.f.=4. (c-d) A similar experiment was conducted in U87EGFR cells with a similar result. (d) Unstained: Erlotinib vs erlotinib+R428: P =0.0045, t=5.71, d.f.=4; Erlotinib vs erlotinib+thalidomide: P =0.0036, t=6.13, d.f.=4. Annexin+PI: Erlotinib vs erlotinib+R428: P =0.0014, t=7.83, d.f.=4; Erlotinib vs erlotinib+thalidomide: P =0.0022, t=6.98, d.f.=4. Data are presented as mean±s.e.m; *P<0.05, **P<0.01, ***P<0.001 using two-tailed unpaired Student's t-test (n =3 biologically independent experimental replicates).
Supplementary Figure 6 Etanercept renders glioma cells sensitive to EGFR inhibition.
(a-b) GBM9 cells were treated with erlotinib and/or enbrel (1μM) for 72 hours followed by Annexin-FACS assay. Unstained cells represent viable cells. Annexin positive cells are undergoing apoptosis. PI (Propidium iodide) positive cells are undergoing late apoptosis. In “Control” cells are treated with control vehicle (DMSO). The combination treatment decreases the number of viable cells and there is an increase in double stained cells (Annexin+PI Positive). (b) Unstained: Erlotinib vs erlotinib+Enbrel: P =0.0008, t=9.11, d.f.=4; Annexin+PI: Erlotinib vs erlotinib+Enbrel: P=0.0012, t=8.18, d.f.=4. (c-d) A similar experiment was conducted in U87EGFRwt cells with a similar result. (d) Unstained: Erlotinib vs erlotinib+Enbrel: P =0.0006, t=9.6, d.f.=4; Annexin+PI: Erlotinib vs erlotinib+Enbrel: P =0.0003, t=11.34, d.f.=4. Data are presented as mean±s.e.m; **P<0.01, ***P<0.001 using two-tailed unpaired Student's t-test (n =3 biologically independent experimental replicates).
Supplementary Figure 7 Combination treatment increases caspase 3/7 activity in glioma cells.
(a) GBM9 cells were treated by erlotinib and/or SP600125, U1026 for 72 hours followed by Caspase-Glo 3/7 Assay. Caspase3/7 activity was evaluated in GBM9 cells exposed to erlotinib plus SP600125. Similar results were obtained using a combination of erlotinib and U1026. Erlotinib vs erlotinib+SP600125: P =0.001, t=8.66, d.f.=4; Erlotinib vs erlotinib+U0126: P =0.0002, t=13.24, d.f.=4. Similar procedures were performed in b-f and similar results were obtained. (b) Erlotinib vs erlotinib+R428: P =0.0004, t=10.76, d.f.=4; Erlotinib vs erlotinib+thalidomide: P=0.0004, t=10.64, d.f.=4. (c) Erlotinib vs erlotinib+SP600125: P<0.0001, t=21.41, d.f.=4; Erlotinib vs erlotinib+U1026: P =0.0007, t=9.49, d.f.=4. (d) Erlotinib vs erlotinib+R428: P=0.0001, t=14.8, d.f.=4; Erlotinib vs erlotinib+T: P <0.0001, t=16.77, d.f.=4. (e) Erlotinib vs erlotinib+enbrel: P =0.0096, t=8.72, d.f.=4. (f) Erlotinib vs erlotinib+enbrel: P =0.0017, t=7.43, d.f.=4. Data are presented as mean±s.e.m; **P<0.01, ***P<0.001, ****P<0.0001 from two-tailed unpaired Student's t-test (n =3 biologically independent experimental replicates).
Supplementary Figure 8 Combination treatment inhibits glioma cell proliferation.
(a) GBM9 cells were plated in 6 well plated and treated with drugs as indicated. Cell proliferation was measured through cell counting using the trypan blue dye exclusion test. A combination of erlotinib and SP600125 (SP) significantly reduces cell growth after 72 hours. Similar results were shown using a combination of erlotinb and U1026 (U). Erlotinib vs erlotinib+SP: P =0.0016, t=7.60, d.f.=4; Erlotinib vs erlotinib+U: P =0.0012, t=8.24, d.f.=4. Similar procedures were performed in b-f and similar results were obtained. (b) Erlotinib vs erlotinib+R428: P =0.0008, t=9.24, d.f.=4; Erlotinib vs erlotinib+T: P =0.0004, t=11.18, d.f.=4. (c) Erlotinib vs erlotinib+SP: P =0.0004, t=10.78, d.f.=4; Erlotinib vs erlotinib+U: P =0.0014, t=7.85, d.f.=4. (d) Erlotinib vs erlotinib+R428: P =0.001, t=8.62, d.f.=4; Erlotinib vs erlotinib+T: P =0.0003, t=11.58, d.f.=4. (e) Erlotinib vs erlotinib+enbrel: P =0.0014, t=7.85, d.f.=4. (f) Erlotinib vs erlotinib+enbrel: P =0.0005, t=10.37, d.f.=4. SP: SP600125; U:U0126; T: Thalidomide. Data are presented as mean±s.e.m; **P<0.01, ***P<0.001 from two-tailed unpaired Student's t-test (n =3 biologically independent experimental replicates).
Supplementary Figure 9 TNF inhibition sensitizes mouse tumors to EGFR inhibition with afatinib in vivo.
(a) Combined treatment of afatinib and thalidomide prolonged survival and suppressed tumor growth in an orthotropic model. Kaplan-Maier survival curves were calculated using GraphPad Prism 7. Statistical significance verified by the log rank test, P=0.0015,**P<0.01. (b) Representative bioluminescence images from afatinib and afatinib plus thalidomide group at day 1, 10 and 20 post-treatment. Since all mice in vehicle and thalidomide group died within 20 days after transplant, images at day 20 post-treatment were not available. (c-d) Body weight of xenograft mice were monitored regularly and recorded every 2 days. No significant change of body weight was observed in subcutaneous mouse model between groups. (e) Body weight of orthotopic models. Body weight losses are found in vehicle, erlotinib and thalidomide group but not in combination therapy (erlotinib+thalidomide). Erlotinib vs erlotinib+thalidmoide: P<0.0001, t=6.32, d.f.=14; (f) Similar results were obtained in orthotopic models treated by afatinib and/or thalidmide. Afatinib vs afatinib+thalidomide: P =0.0003, t=4.79, d.f.=14.
Supplementary Figure 10 Erlotinib treatment activates Axl–JNK–ERK signaling in orthotopic models; this is suppressed by TNF inhibition.
(a) Immunostainig of pAXL, pJNK and pERK proteins from a representative brain section from vehicle, erlotinb and erlotinib plus thalidomide group. Cells with brown staining are considered as positive. Erlotinib group shows higher expression of pAxl, pJNK and pERK compared to vehicle group, whereas thaldimide inhibits erlotinib induced pAxl-pJNK-pERK activation. (b-d) Semi-quantitative analysis of pAxl, pJNK and pERK immunostaining. Four random fields in 3 tissue blocks at x200 magnification were scored semiquantitatively as: 0=No positive staining; 1=1-25% tumor cells stained, 2=26%-75% tumor cells stained and 3=>75% tumor cells stained. Differences between treatment groups were analyzed by the Mann-Whitney U test (n=12). (b) pAXL: Erlotinib vs. vehicle, p=0.0062, U=26; Erlotinib vs. erlotinib+thalidomide, p=0.0093, U=28. (c) pJNK: Erlotinib vs. vehicle, p=0.022, U=32; Erlotinib vs. erlotinib+thalidomide, p=0.014, U=29.5. (d) pERK: Erlotinib vs. vehicle, p=0.0040, U=23.5; Erlotinib vs. erlotinib+thalidomide, p=0.0026, U=21.
Supplementary Figure 11 Full-length western blots.
Full-length Western blots for cropped images in Fig. 1.
Supplementary Figure 12 Full-length western blots and DNA agarose gel.
Full-length Western blots for cropped images in Fig. 2 and Fig. 3f, 3h, 3i.
Supplementary Figure 13 Full-length western blots.
Full-length Western blots for cropped images in Fig. 4g, 4h, 4i.
Supplementary Figure 14 Full-length western blots.
Full-length Western blots for cropped images in Fig. 6j-l and Fig. 7f, 7g.
Supplementary Figure 15 Full-length western blots.
Full-length Western blots for cropped images in Supplementary Fig. 1 and 2d,e,g–i.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15. (PDF 3126 kb)
Rights and permissions
About this article
Cite this article
Guo, G., Gong, K., Ali, S. et al. A TNF–JNK–Axl–ERK signaling axis mediates primary resistance to EGFR inhibition in glioblastoma. Nat Neurosci 20, 1074–1084 (2017). https://doi.org/10.1038/nn.4584
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4584
This article is cited by
-
Endosome associated trafficking regulator 1 promotes tumor growth and invasion of glioblastoma multiforme via inhibiting TNF signaling pathway
Journal of Neuro-Oncology (2024)
-
Dual blockade of EGFR and PI3K signaling pathways offers a therapeutic strategy for glioblastoma
Cell Communication and Signaling (2023)
-
Deletion of DYRK1A Accelerates Osteoarthritis Progression Through Suppression of EGFR-ERK Signaling
Inflammation (2023)
-
Protein tyrosine kinase inhibitor resistance in malignant tumors: molecular mechanisms and future perspective
Signal Transduction and Targeted Therapy (2022)
-
EGFR ligand shifts the role of EGFR from oncogene to tumour suppressor in EGFR-amplified glioblastoma by suppressing invasion through BIN3 upregulation
Nature Cell Biology (2022)