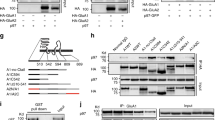
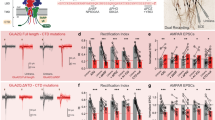
Abstract
Plasticity in the brain is essential for maintaining memory and learning and is associated with the dynamic membrane trafficking of AMPA receptors. EphrinB proteins, ligands for EphB receptor tyrosine kinases, are transmembrane molecules with signaling capabilities that are required for spine morphogenesis, synapse formation and synaptic plasticity. Here, we describe a molecular mechanism for ephrinB2 function in controlling synaptic transmission. EphrinB2 signaling is critical for the stabilization of AMPA receptors at the cellular membrane. Mouse hippocampal neurons from conditional ephrinB2 knockouts showed enhanced constitutive internalization of AMPA receptors and reduced synaptic transmission. Mechanistically, glutamate receptor interacting proteins bridge ephrinB ligands and AMPA receptors. Moreover, this function involved a regulatory aspect of ephrinB reverse signaling that involves the phosphorylation of a single serine residue in their cytoplasmic tails. In summary, our findings uncover a model of cooperative AMPA receptor and ephrinB reverse signaling at the synapse.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Hayashi, Y. et al. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287, 2262–2267 (2000).
Beattie, E.C. et al. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat. Neurosci. 3, 1291–1300 (2000).
Bredt, D.S. & Nicoll, R.A. AMPA receptor trafficking at excitatory synapses. Neuron 40, 361–379 (2003).
Shi, S., Hayashi, Y., Esteban, J.A. & Malinow, R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105, 331–343 (2001).
Lee, S.H., Liu, L., Wang, Y.T. & Sheng, M. Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron 36, 661–674 (2002).
Plant, K. et al. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat. Neurosci. 9, 602–604 (2006).
Cowan, C.A. & Henkemeyer, M. The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature 413, 174–179 (2001).
Lu, Q., Sun, E.E., Klein, R.S. & Flanagan, J.G. Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G protein–coupled chemoattraction. Cell 105, 69–79 (2001).
Palmer, A. et al. EphrinB phosphorylation and reverse signaling: regulation by Src kinases and PTP-BL phosphatase. Mol. Cell 9, 725–737 (2002).
Palmer, A. & Klein, R. Multiple roles of ephrins in morphogenesis, neuronal networking and brain function. Genes Dev. 17, 1429–1450 (2003).
Segura, I., Essmann, C.L., Weinges, S. & Acker-Palmer, A. Grb4 and GIT1 transduce ephrinB reverse signals modulating spine morphogenesis and synapse formation. Nat. Neurosci. 10, 301–310 (2007).
Grunwald, I.C. et al. Hippocampal plasticity requires postsynaptic ephrinBs. Nat. Neurosci. 7, 33–40 (2004).
Bouzioukh, F. et al. Tyrosine phosphorylation sites in ephrinB2 are required for hippocampal long-term potentiation but not long-term depression. J. Neurosci. 27, 11279–11288 (2007).
Man, H.Y. et al. Regulation of AMPA receptor–mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron 25, 649–662 (2000).
Lin, J.W. et al. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat. Neurosci. 3, 1282–1290 (2000).
Ehlers, M.D. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron 28, 511–525 (2000).
Torres, R. et al. PDZ proteins bind, cluster and synaptically colocalize with Eph receptors and their ephrin ligands. Neuron 21, 1453–1463 (1998).
Brückner, K. et al. EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron 22, 511–524 (1999).
Dong, H. et al. GRIP: a synaptic PDZ domain–containing protein that interacts with AMPA receptors. Nature 386, 279–284 (1997).
Kim, C.H., Chung, H.J., Lee, H.K. & Huganir, R.L. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc. Natl. Acad. Sci. USA 98, 11725–11730 (2001).
Wyszynski, M. et al. Interaction between GRIP and liprin-α/SYD2 is required for AMPA receptor targeting. Neuron 34, 39–52 (2002).
Chung, H.J., Steinberg, J.P., Huganir, R.L. & Linden, D.J. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science 300, 1751–1755 (2003).
Lu, W. & Ziff, E.B. PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking. Neuron 47, 407–421 (2005).
Barria, A. et al. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science 276, 2042–2045 (1997).
Lee, H.K. et al. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature 405, 955–959 (2000).
Whitlock, J.R., Heynen, A.J., Shuler, M.G. & Bear, M.F. Learning induces long-term potentiation in the hippocampus. Science 313, 1093–1097 (2006).
Makinen, T. et al. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 19, 397–410 (2005).
Zamanillo, D. et al. Importance of AMPA receptors for hippocampal synaptic plasticity, but not for spatial learning. Science 284, 1805–1811 (1999).
Rouach, N. et al. TARP γ8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nat. Neurosci. 8, 1525–1533 (2005).
Kayser, M.S., McClelland, A.C., Hughes, E.G. & Dalva, M.B. Intracellular and trans-synaptic regulation of glutamatergic synaptogenesis by EphB receptors. J. Neurosci. 26, 12152–12164 (2006).
Chung, H.J. et al. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain–containing proteins. J. Neurosci. 20, 7258–7267 (2000).
Daw, M.I. et al. PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses. Neuron 28, 873–886 (2000).
Matsuda, S., Launey, T., Mikawa, S. & Hirai, H. Disruption of AMPA receptor GluR2 clusters following long-term depression induction in cerebellar Purkinje neurons. EMBO J. 19, 2765–2774 (2000).
Osten, P. et al. Mutagenesis reveals a role for ABP/GRIP binding to GluR2 in synaptic surface accumulation of the AMPA receptor. Neuron 27, 313–325 (2000).
Xia, J. et al. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain–containing proteins. Neuron 28, 499–510 (2000).
Zimmer, M., Palmer, A., Kohler, J. & Klein, R. EphB-ephrinB bi-directional endocytosis terminates adhesion allowing contact-mediated repulsion. Nat. Cell Biol. 5, 869–878 (2003).
Fu, J., deSouza, S. & Ziff, E.B. Intracellular membrane targeting and suppression of Ser880 phosphorylation of glutamate receptor 2 by the linker I-set II domain of AMPA receptor–binding protein. J. Neurosci. 23, 7592–7601 (2003).
Brambilla, R. et al. Membrane-bound LERK2 ligand can signal through three different Eph-related receptor tyrosine kinases. EMBO J. 14, 3116–3126 (1995).
Sambrook, J., Fritsch, E.F. & Maniatis, T. Molecular Cloning: A Laboratory Manual 2nd edn (Cold Spring Harbor Laboratory Press, New York, 1989).
Palmer, A., Gavin, A.C. & Nebreda, A.R. A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. EMBO J. 17, 5037–5047 (1998).
Acknowledgements
We would like to thank A.Yamasaki for technical help, J. Lauterbach and G. Wilkinson for valuable reagents, I. Kadow for helpful discussions, and A.Yamasaki, E. Fellows, I. Kadow, T. Acker and S. Sigrist for critically reading the manuscript. This work was supported by grants from the Deutsche Forschungsgemeinschaft (AC180/2-1 and AC180/2-2 to A.A.-P.) and the Cluster of Excellence 'Macromolecular Complexes' at the Goethe University Frankfurt (EXC 115).
Author information
Authors and Affiliations
Contributions
All the antibody feeding assays, surface biotinylation assays, colocalization and surface clustering experiments were carried out by C.L.E. with the help of E.M. and J.C.G. M.Z. and E.M. performed the biochemical and cellular characterization of GRIP binding to ephrinB ligands. R.K. supervised and supported the work of M.Z. Eph-GRIP–binding experiments were carried out by E.M. M.H.T. performed the electrophysiology under the supervision of V.S. A.A.-P. carried out the kinase assays, designed and supervised the experiments, and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Methods (PDF 1031 kb)
Rights and permissions
About this article
Cite this article
Essmann, C., Martinez, E., Geiger, J. et al. Serine phosphorylation of ephrinB2 regulates trafficking of synaptic AMPA receptors. Nat Neurosci 11, 1035–1043 (2008). https://doi.org/10.1038/nn.2171
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2171
This article is cited by
-
Digenic inheritance of mutations in EPHA2 and SLC26A4 in Pendred syndrome
Nature Communications (2020)
-
AMPA receptors and their minions: auxiliary proteins in AMPA receptor trafficking
Cellular and Molecular Life Sciences (2019)
-
EphrinB2 repression through ZEB2 mediates tumour invasion and anti-angiogenic resistance
Nature Communications (2016)
-
CDKL5 ensures excitatory synapse stability by reinforcing NGL-1–PSD95 interaction in the postsynaptic compartment and is impaired in patient iPSC-derived neurons
Nature Cell Biology (2012)
-
A dual shaping mechanism for postsynaptic ephrin-B3 as a receptor that sculpts dendrites and synapses
Nature Neuroscience (2011)