Abstract
The development of a tuberculosis (TB) vaccine that induces sterilizing immunity to Mycobacterium tuberculosis infection has been elusive. Absence of sterilizing immunity induced by TB vaccines may be due to delayed activation of mucosal dendritic cells (DCs), and subsequent delay in antigen presentation and activation of vaccine-induced CD4+ T-cell responses. Here we show that pulmonary delivery of activated M. tuberculosis antigen-primed DCs into vaccinated mice, at the time of M. tuberculosis exposure, can overcome the delay in accumulation of vaccine-induced CD4+ T-cell responses. In addition, activating endogenous host CD103+ DCs and the CD40–CD40L pathway can similarly induce rapid accumulation of vaccine-induced lung CD4+ T-cell responses and limit early M. tuberculosis growth. Thus, our study provides proof of concept that targeting mucosal DCs can accelerate vaccine-induced T-cell responses on M. tuberculosis infection, and provide insights to overcome bottlenecks in TB vaccine efficacy.
Similar content being viewed by others
Introduction
Tuberculosis (TB) is a leading cause of death by infection1. TB is caused by aerosol exposure to the intracellular bacterium Mycobacterium tuberculosis (Mtb), leading to either latent disease or active pulmonary disease. The only currently licensed vaccine against TB is Mycobacterium bovis Bacille Calmette–Guerin (BCG). Although BCG vaccination is effective against childhood forms of TB1, and in decreasing childhood TB morbidity1, it provides variable efficacy against adult pulmonary TB. Thus, over the past two decades, concerted efforts have been made to develop new vaccines for TB that will provide improved protection on Mtb exposure. Modern candidate TB vaccines have focussed on induction of T-cell responses, primarily CD4+ T cells producing interferon gamma (IFNγ)2. More recently, an important role for mucosal interleukin 17A (IL-17A) in vaccine-induced protection against TB disease has been shown3,4,5,6. Thus, induction of lung-resident IL-17A-producing CD4+ T-cell populations by TB vaccines is also being explored2,4,5,7. Despite these efforts, most TB vaccines reduce the burden of lung Mtb by ∼0.5–1.0 log in animal challenge models3,8,9,10,11. Recombinant live mycobacterial vaccines confer improved protection (∼2 log reduction), when compared with subunit and virally vectored TB vaccines. Examples of recombinant vaccines include the recombinant Mycobacterium smegmatis vaccine, which induces sterilizing immunity in the liver, but not the lung12; recombinant BCG over-expressing Listeria monocytogenes listeriolysin and lacking urease C11,13; and the recombinant Mtb vaccine lacking PhoP14. Other work has highlighted the benefit of administering recombinant Mtb vaccines mucosally, showing that macaques vaccinated with the attenuated Mtb mutant lacking SigH had sterile protection in some TB lesions15. Although these results are promising, considerable challenges are associated with the design and implementation of a recombinant Mtb vaccine to be delivered mucosally via the lungs, particularly in light of the TB–HIV co-epidemic. Thus, it is critical to fully understand the early events occurring in the vaccinated Mtb-infected lung.
After Mtb infection of naive mice, accumulation of activated lung CD4+ T cells is delayed, occurring between 14 and 21 days post Mtb infection (dpi)16,17. This delay is thought to be due to Mtb-mediated inhibition of early antigen presentation by antigen-presenting cells (APCs)18,19,20. Even in the presence of an existing Mtb-specific vaccine-induced CD4+ T-cell population in the lung, CD4+ T-memory cells do not accumulate in the lung until 12–14 dpi3,6,21. Priming of T-cell responses following primary Mtb infection requires trafficking of Mtb-infected DCs from the lungs to the lymph nodes (LNs)20,22. Furthermore, Mtb-infected DCs do not efficiently present antigen directly to Mtb-specific CD4+ T cells, but antigen is transferred from Mtb-infected DCs to uninfected bystander DCs in the LNs, for antigen presentation to naive CD4+ T cells22. On Mtb infection, even vaccine-induced memory T cells accumulate in the LNs before mobilization to the lungs6. Thus, in the current study, we hypothesized that the delay in accumulation of lung vaccine-induced CD4+ T cells in vaccinated Mtb-infected hosts is due to a delay in antigen presentation by Mtb-infected APCs, thus resulting in a bottleneck and preventing sterilizing immunity to Mtb infection. We show that we can overcome the delay in accumulation of vaccine-induced memory CD4+ T cells by transferring exogenously primed activated DCs into the lungs of vaccinated mice at the time of Mtb infection. DC transfer into vaccinated Mtb-infected hosts results in rapid activation of vaccine-induced CD4+ T-cell responses, substantial changes in the lung micro-environment, activation of lung alveolar macrophages and early Mtb control. Furthermore, these protective mechanisms are dependent on CD103+ DCs and the CD40–CD40L activation pathway, as host-directed therapeutics targeting these pathways in vaccinated Mtb-infected mice can mimic the protective effects of pulmonary DC transfer. Thus, we have determined a key bottleneck for the failure of TB vaccines to induce sterilizing protection against Mtb infection. These results provide a roadmap for the type of immune responses that a sterilizing TB vaccine should induce, representing a milestone in our mechanistic understanding of TB vaccine-mediated immune responses.
Results
DC transfer confers superior vaccine-induced Mtb control
Following Mtb infection, vaccine-induced CD4+ T-cell responses are delayed in vaccinated hosts3,6, and could be a likely reason for the failure of TB vaccines to induce sterilizing immunity. Therefore, we first assessed whether a delay in accumulation of CD4+ T-cell recall responses was due to an inherent deficiency in the ability of the vaccine-induced T cells to respond to antigen exposure. Thus, naive CD4+ T cells were isolated from Mtb-specific immunodominant antigen 85B (Ag85B) T-cell receptor transgenic (TCR) mice and adoptively transferred into congenic C57BL/6 (B6) mice, which were vaccinated subcutaneously (s.c.) with BCG, rested for 4 weeks followed by a mucosal boost with the CD4+ T-cell epitope Ag85B240–254. Following a period of rest for 4 weeks, lung CD4+ T cells were isolated and cultured in vitro in the presence of Ag85B-pulsed DCs. Recall Ag85B-specific CD4+ T cells proliferated rapidly, underwent activation, produced cytokines and on co-culture with Mtb-infected macrophages could mediate Mtb control (Fig. 1a–d). These data suggest that vaccine-induced CD4+ T cells can rapidly respond to Mtb antigen, but activation is delayed in vivo following Mtb infection3,6,21. This is not due to a limitation of Mtb antigen availability, as infection of vaccinated mice with high doses (∼1,000 c.f.u.) of Mtb did not improve vaccine-induced Mtb control (Supplementary Fig. 1a)23. T regulatory cells induced in response to Mtb infection are known to suppress early T-cell responses24. As before25, however, depletion of T regulatory cells in vaccinated B6 mice early following Mtb infection did not improve vaccine-induced Mtb control, instead increasing susceptibility to Mtb infection (Supplementary Fig. 1b). Thus, although recall CD4+ T-cell responses are delayed in vivo in Mtb-infected hosts3,6,21, vaccine-induced CD4+ T cells can rapidly respond ex vivo to activate macrophages for Mtb control.
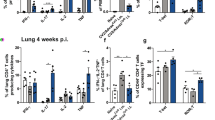
(a–d) Naive CD4+ T cells from Ag85B TCR Tg Thy1.1+ mice were transferred into B6 mice and were vaccinated with BCG s.c. followed by mucosal boost with Ag85B240–254 peptide in mucosal adjuvant, rested for 4 weeks, before isolating total lung cells and labelling with CFSE. Cells were cultured for 3 days with either Ag85B antigen alone with or without Mtb, or BMDC pulsed with Ag85B antigen. (a) Expansion and (b) proliferation of Mtb-specific cells was assessed by flow cytometry and CFSE dilution. (c) Cytokine production was measured in supernatants. However some cytokine levels are not visible on the scale used. (d) Six day-stimulated T cells were cultured with Mtb-infected BMDMs additional 6 days and intracellular Mtb c.f.u. determined by plating. (e–i) B6 mice were vaccinated with BCG s.c. followed by mucosal boost with Ag85B240–254 peptide in mucosal adjuvant, rested for 4 weeks and infected with ∼100 c.f.u. Mtb HN878. Vaccinated mice received Ag85B-Z-DC on −1 and 4 dpi. Lungs were collected and flow cytometry was used to assess (e) CD44 expression on CD3+CD4+ T cells, and (f) IL-17 and IFN-γ production by Ag85B-specific CD4+ CD44hi T cells in the lungs. (g) Flow cytometry was used to assess MHC-II mean fluorescence intensity (MFI) on lung alveolar macrophages, and fold change relative to unvaccinated was calculated. A representative plot of MHC-II expression on alveolar macrophages from unvaccinated, vaccinated and vaccinated mice receiving Z-DC transfer is shown at 8 dpi. (h) Lung bacterial burden was determined by plating. (i) B-cell lymphoid follicle formation was determined by CD3 (red) and B220 (green) staining on formalin-fixed, paraffin-embedded sections by immunofluorescence staining. The average size of B-cell follicles per lobe was quantified using the automated tool of the Zeiss Axioplan 2 microscope in squared microns, representative images of B-cell follicles are shown. (a–d) n=3–4 technical replicates±s.d., (e–i) n=4–5 biological replicates±s.d. *P≤0.05, **P≤0.01, ***P≤0.001 by one-way analysis of variance (ANOVA) (a–d) or two-way ANOVA (e–i). ND, not detected. Dotted lines on h represent the limit of detection by plating.
To overcome the delayed activation of CD4+ T-cell recall responses on Mtb infection in vivo, we addressed whether delivery of Mtb antigen-pulsed DCs into the lungs of Mtb-infected-vaccinated mice could overcome this bottleneck and improve recall vaccine-induced CD4+ T-cell immunity. B6 mice were thus vaccinated with BCG, rested for 4 weeks, followed by a mucosal boost with Ag85B240–254. Mice were further rested for 4 weeks, after which they were challenged with a clinical W-Beijing Mtb strain, HN878 (ref. 26). At the time of Mtb challenge, vaccinated mice received Ag85B240–254-pulsed B6 bone marrow-derived DCs (BMDC) delivered intratracheally (i.t., DC transfer). While vaccination and mucosal boost reduced lung Mtb burden as before4, B6 DC transfer into vaccinated mice reduced lung Mtb burden by ∼98.6%, when compared with the burden in unvaccinated Mtb-infected mice (Supplementary Fig. 2a,b). Pulmonary delivery of Ag85B240–254 peptide alone did not induce the superior protection seen with DC transfer in vaccinated mice (Supplementary Fig. 2a), further suggesting that antigen availability was not the major reason for delayed vaccine-induced CD4+ T-cell recall responses. To further improve the ability of antigen-pulsed DCs to activate CD4+ T cells following Mtb infection, we next stimulated DCs with the Dectin-1 agonist zymosan (Z-DC)27 at the time of antigen pulsing. Activating Z-DC, induced proinflammatory cytokines such as IL-23, IL-1β and IL-6 (Supplementary Fig. 3a–c), but also induced IL-10 (Supplementary Fig. 3d). IL-10 induction can dampen vaccine-induced immunity to Mtb infection28,29, and we next tested whether use of B6 DCs or Il10−/− DCs treated with zymosan was more effective on transfer at conferring Mtb control in vaccinated hosts. B6 DC transfer into vaccinated Mtb-infected mice as before reduced Mtb burden significantly, when compared with vaccinated Mtb-infected hosts, while transfer of B6 Z-DCs had a small but significant improvement in Mtb control, when compared with vaccinated hosts receiving B6 DC transfer (Supplementary Fig. 2b). In sharp contrast, we found that vaccinated hosts receiving Z-DCs from Il10−/− mice resulted in far superior Mtb control, with Mtb burden-bordering limit of detection by plating assays (Supplementary Fig. 2b, limit of Mtb detection is ∼75–100 c.f.u. per lung, Mtb burden from three out of five mice were in this range, ∼99.97% reduction in Mtb burden, when compared with unvaccinated Mtb-infected mice). The near-sterilizing protection in vaccinated mice receiving Il10−/− Z-DC transfer coincided with increased frequency of both activated IL-17-producing and IFNγ-producing Mtb-specific CD4+ T cells in the lungs (Supplementary Fig. 2c,d; gating strategy in Supplementary Fig. 4a). Indeed, B6 Z-DC transfer also resulted in increased frequency of lung IL-17-producing Mtb-specific CD4+ T-cell responses, but failed to improve IFNγ-producing CD4+ T-cell responses, when compared with responses in vaccinated hosts (Supplementary Fig. 2c,d). These results together suggest that Z-DCs generated from Il10−/− mice on transfer into vaccinated hosts can overcome a TB vaccine bottleneck, and confer superior protection against Mtb infection by significantly accelerating the accumulation of Mtb-specific cytokine-producing CD4+ T-cell vaccine responses. Thus, in all experiments in the remainder of the study, Z-DCs from Il10−/− mice (Ag85B-Z-DC) were used for DC transfer.
DC transfer accelerates vaccine CD4+ T-cell activation
Having established the ability of Z-DC transfer to significantly reduce Mtb burden in the lungs of vaccinated mice, we then assessed the kinetics of immune responses in Mtb HN878-infected vaccinated mice receiving Z-DC transfer. Vaccine-induced CD4+ T-cell responses and activation of lung myeloid cells are not detected in vaccinated hosts until 12–14 dpi following Mtb challenge3. In contrast, we observed significant early accumulation (8 dpi) of activated CD4+ T cells in vaccinated Mtb-infected mice receiving Z-DC transfer, when compared with vaccinated Mtb-infected mice (Fig. 1e; gating strategy in Supplementary Fig. 4a). In addition, increased numbers of Mtb-specific CD44hi-activated cells producing the cytokines IL-17 and IFN-γ accumulated early in lungs of vaccinated Mtb-infected mice receiving Z-DC transfer, when compared with vaccinated Mtb-infected mice or naive Mtb-infected mice (Fig. 1f). As noted previously, activated T-cell responses in vaccinated mice not receiving Z-DC transfer accumulated later at 14 dpi (Fig. 1f)6, while T-cell responses between the different groups were comparable at later time points. These protective and early activation events in vaccine-induced CD4+ T-cell responses coincided with increased early upregulation of major histocompatibility complex (MHC)-II expression on lung alveolar macrophages in Z-DC-recipient-vaccinated Mtb-infected mice, when compared with levels expressed in unvaccinated mice (Fig. 1g; gating strategy in Supplementary Fig. 4b). Importantly, the early and rapid activation of vaccine-induced CD4+ T-cell responses, and concomitant activation of alveolar macrophages was associated with complete control of Mtb growth, maintaining burdens at levels of initial inoculum up to 20 dpi in vaccinated Mtb-infected mice receiving Z-DC transfer (Fig. 1h). This is in contrast to naive and vaccinated Mtb-infected mice, in which Mtb burden increased in the lungs over time (Fig. 1h). Finally, Z-DC transfer at the time of Mtb challenge resulted in long-lasting protection up to 40 dpi, with Z-DC transfer-recipient mice still maintaining significantly lower bacterial burdens in the lungs (Fig. 1h). As expected, at later time points, vaccinated mice showed similar Mtb burden as unvaccinated mice30, suggesting that while Z-DC transfer provides long-lasting vaccine-induced immunity in Mtb HN878-infected mice, endogenous vaccine-induced responses are not long-lasting and are lost over time. Furthermore, robust rapid formation of B-cell follicles-harbouring localized T cells could be detected in the lungs of vaccinated Mtb-infected mice receiving Z-DC transfer (Fig. 1i), indicating early development of protective lung granulomas. Importantly, even on challenge with a Euro-American Mtb strain, H37Rv, we found that Z-DC transfer effectively conferred superior Mtb control by activating alveolar macrophages and accelerating accumulation of activated CD4+ T cells (Supplementary Fig. 5a–d). Thus, our results suggest that DC transfer can effectively induce superior vaccine control on challenge with different Mtb strains. As Mtb HN878 is a clinically relevant rapidly emerging Mtb strain, all experiments in the remainder of the study was carried out with Mtb HN878. Thus, our data together suggest that DC transfer is effective in rapid restimulation of vaccine-induced T cells to produce cytokines and localize within lymphoid follicles to activate macrophages for rapid Mtb control, which is sustained long term, and across different Mtb strains.
DC transfer-mediated Mtb control is CD4+ T-cell dependent
To test the durability of Z-DC transfer in inducing superior vaccine-induced immunity against Mtb challenge, BCG-vaccinated mice were boosted mucosally and rested for 10 weeks after vaccination, and Z-DC transfer and Mtb HN878 infection were carried out as before. Indeed, we found that Z-DC transfer still induced superior protection in long-term-rested-vaccinated hosts, with Mtb burden significantly lower and at levels of detection, when compared with control vaccinated Mtb-infected mice that were also rested for 10 weeks before Mtb challenge (Supplementary Fig. 6a). Importantly, vaccinated mice receiving Z-DC transfer also showed increased accumulation of activated IL-17+ and IFN-γ+ CD4+ T cells, when compared with CD4+ T-cell responses in vaccinated and unvaccinated Mtb-infected mice (Supplementary Fig. 6b,c). These data suggest that Z-DC transfer-induced protection confers superior Mtb control even following a prolonged period of rest, supporting the durability of DC transfer-mediated protective efficacy in overcoming the TB vaccine bottleneck.
Previous studies have employed administration of DCs as vaccination strategy31,32, during which mice were immunized with antigen-primed DCs, and then rested before Mtb infection. In contrast, the approach taken here is to enhance antigen presentation at the time of Mtb infection by administering DCs mucosally at the time of Mtb infection. Thus, we tested whether use of Z-DCs as a mucosal boost to BCG vaccination, would similarly induce superior Mtb control. Indeed, we show that pulmonary administration of Z-DCs as a mucosal boost to BCG vaccination only had a small protective effect in vaccinated hosts, and did not match the near-sterilizing Mtb control seen, when Z-DC transfer was carried out at the time of Mtb infection in vaccinated hosts (Supplementary Fig. 6d). These data show that the improved protection conferred by Z-DC transfer is specifically due to the enhanced antigen presentation and accelerated T cells priming at the time of Mtb infection, and use of DCs as vaccination tool does not enable the superior Mtb control in vaccinated hosts.
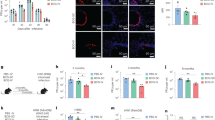
Our data show that DC transfer into vaccinated mice at the time of Mtb challenge accelerates Mtb-specific CD4+ T-cell responses and confers superior protection against Mtb challenge. Thus, we next addressed whether DC transfer-induced immunity in vaccinated hosts was dependent on vaccine-induced T-cell responses, or whether DC transfer will similarly induce near-sterilizing immunity in previously unvaccinated Mtb-infected mice by accelerating priming of naive CD4+ T-cell responses. Thus, unvaccinated B6 mice or B6 mice that were BCG vaccinated and mucosally boosted were rested for 4 weeks, and then received Z-DC transfer at the time of Mtb challenge, and Mtb control was determined. Our data show that while vaccinated Mtb-infected mice receiving Z-DC demonstrated superior bacterial control as before, Mtb control in unvaccinated mice receiving Z-DC transfer was less effective and resembled Mtb control seen in vaccinated hosts not receiving Z-DC transfer (Fig. 2a). Furthermore, although the numbers of IL-17 and IFN-γ-producing Mtb-specific CD4+ T cells in Z-DC transfer-recipient Mtb-infected mice were significantly higher, when compared with Mtb-infected mice not receiving Z-DC, the numbers were reduced, when compared with numbers seen in vaccinated mice receiving Z-DC transfer (Fig. 2b,c). In addition, T-cell localization within B-cell follicles in vaccinated mice receiving Z-DC transfer was higher, when compared with unvaccinated mice that received Z-DC transfer (Fig. 2d). These results together suggest that DC transfer into unvaccinated hosts can also accelerate the priming of naive CD4+ T cells into cytokine-producing Mtb-specific CD4+ T-cell responses and improve Mtb control. However, the protection in naive Mtb-infected mice receiving Z-DC transfer is not as effective as seen in vaccinated mice receiving Z-DC transfer, suggesting that Z-DC transfer mediating near-sterilizing immunity occurs only in previously vaccinated hosts.
(a–d) Unvaccinated B6 mice, or B6 mice vaccinated with BCG s.c. followed by mucosal boost with Ag85B240–254 peptide in mucosal adjuvant, were rested for 4 weeks, and Mtb HN878-infected (∼100 c.f.u.) and treated with Ag85B-Z-DC on −1 and 4 dpi, and (a) bacterial burden was assessed by plating at 20 dpi. Flow cytometry was used to determine production of (b) IL-17 and (c) IFN-γ by Ag85B-specific CD4+CD44hi T cells. (d) B-cell lymphoid follicle formation was determined by CD3 (red) and B220 (green) staining on formalin-fixed, paraffin-embedded sections by immunofluorescence staining on 20 dpi. The average size of B-cell follicles per lobe was quantified using the morphometric tool of the Zeiss Axioplan. (e–g) B6 mice were vaccinated as above, and infected with Mtb HN878 (∼100 c.f.u.) via aerosol. Mice received Ag85B-Z-DC transfer on −1 and 4 dpi; and were treated with 300 μg GK1.5 (anti-CD4) or isotype control delivered i.p. on 0 and 7 dpi. Lungs were collected at 20 dpi. (e) Lung bacterial burden was determined by plating. Flow cytometry was used to assess (f) IL-17 and (g) IFN-γ production by Ag85B-specific CD4+CD44hi T cells. (a–d) n=8–10 biological replicates±s.d., (e–g) n=5–10 biological replicates±s.d., *P≤0.05, **P≤0.01, ***P≤0.001 by one-way analysis of variance or Kruskall–Wallis test (e) or Student’s t-test (d) (UnVac+Ag85B-Z-DC compared with Vac+Ag85B-Z-DC). Dotted lines on bacterial burden plots represent the limit of detection by plating.
To further confirm that DC transfer-mediated protection is vaccine-induced CD4+ T-cell-dependent, vaccinated mice receiving Z-DC transfer received a CD4 depleting antibody at early time points following Mtb challenge, and Mtb control in vaccinated hosts receiving Z-DC transfer was assessed at 20 dpi. Our data show that early depletion of CD4+ T cells in vaccinated mice receiving DC transfer reversed the superior protection (Fig. 2e) and coincided with loss of early accumulation of IFN-γ- and IL-17-producing Mtb-specific CD4+ T-cell responses (Fig. 2f,g). These results together project that the near-sterilizing protection seen on DC transfer in vaccinated host is dependent on the presence of vaccine-induced CD4+ T-cell responses, as either absence of vaccination or absence of CD4+ T cells abrogates the superior near-sterilizing protection.
DC transfer overcomes bottlenecks in different vaccinations
Our data show that Z-DC transfer accelerates vaccine-induced Mtb-specific CD4+ T-cell responses in mice vaccinated with BCG and mucosally boosted with Ag85B240–254 to confer superior protection on Mtb challenge. Thus, we next tested whether Z-DC transfer could be broadly used to provide superior protection in different vaccination strategies. Recent work by us and others has highlighted the importance of a lung-resident T-cell responses following mucosal vaccination in protection against TB disease4,5. In addition, mucosal vaccination induces potent lung-resident CD4+ T-cell responses, while standard parenteral BCG vaccination induces potent systemic vaccine-induced CD4+ T-cell responses (Supplementary Fig. 7a,b). Thus, we assessed whether Z-DC transfer would improve Mtb protection when used in standard BCG vaccination, when compared with mice that only received mucosal vaccination. B6 mice were vaccinated with either a mucosal TB vaccine consisting of Ag85B240–254 in mucosal adjuvant4 or parenterally with BCG28, rested, and on Mtb challenge received Z-DC transfer. Z-DC transfer provided superior vaccine-induced Mtb control in both mucosally vaccinated and BCG-vaccinated mice (Fig. 3a), suggesting that DC transfer functions broadly in different vaccination strategies. We found that while Z-DC transfer in mucosally vaccinated mice induced accumulation of large numbers of IL-17-producing Mtb-specific CD4+ T-cell responses, IFN-γ-producing CD4+ T-cell responses were not significantly increased (Fig. 3b,c). In contrast, BCG-vaccinated mice receiving DC transfer exhibited increased both IFN-γ and IL-17 CD4+ T-cell responses (Fig. 3b,c). Indeed, the improved CD4+ T-cell recall responses in vaccinated hosts receiving Z-DC transfer coincided with enhanced activation of alveolar macrophages when compared to unvaccinated hosts (Fig. 3d). Thus, our data show that although the effector cytokine mechanism by which Z-DC transfer confers superior protection in vaccination models may be different, Z-DC transfer can rapidly activate both systemic and mucosal CD4+ T-cell responses to induce superior protection against Mtb control in different vaccine strategies and is broadly applicable.
Mice were mucosally vaccinated with three doses of Ag85B240–254 peptide in mucosal adjuvant, or parenterally vaccinated with BCG s.c., rested for 4 weeks, and were Mtb HN878-infected (∼100 c.f.u.) and received Ag85B-Z-DC transfer as described previously. (a) Lung bacterial burden was assessed at 20 dpi by plating. Flow cytometry was used to determine (b) IL-17 and (c) IFN-γ production by Ag85B-specific CD4+ CD44hi T cells. (d) The mean fluorescence intensity (MFI) was calculated to determine level of MHC-II expression on alveolar macrophages, and fold change MFI relative to UnVac was calculated for each group at 20 dpi. n=4–5 biological replicates±s.d. *P≤0.05, **P≤0.01, ***P≤0.001 by one-way analysis of variance or Kruskall–Wallis test (c), or Student’s t-test (d) (BCG Vac compared with BCG Vac+Ag85B-Z-DC). Dotted lines on bacterial burden plots represent the limit of detection by plating.
Identifying host pathways to overcome TB bottlenecks
Transfer of exogenously primed DCs into vaccinated mice can effectively accelerate the vaccine-induced CD4+ T-cell response following Mtb infection, and improve bacterial control. DC transfer as therapy for use in Mtb-infected individuals in TB-endemic areas; however, is logistically difficult to translate for human use as timing of Mtb exposure is impossible to predict. Thus, to identify endogenous host pathways that could be targeted for future use, we carried out transcriptional profiling in lungs of vaccinated Mtb-infected mice receiving DC transfer, and compared it with gene expression profiles in lungs from Mtb-infected-vaccinated mice. Several genes involved in early T-cell activation, macrophage function, as well as chemokines involved in B-cell follicle formation, were significantly upregulated (Supplementary Tables 1 and 2; Fig. 4a; validated in Supplementary Fig. 8). We found that the genes induced rapidly at 8 dpi in vaccinated Mtb-infected mice receiving Z-DC transfer, were not upregulated until 21 dpi in Mtb-infected mice (Fig. 4b)33. These results further support our findings that protective immune responses are substantially accelerated in vaccinated Mtb-infected mice receiving Z-DC transfer.
B6 mice were vaccinated with BCG s.c. followed by mucosal boost with Ag85B240–254 peptide in mucosal adjuvant, rested for 4 weeks, and Mtb HN878-infected (∼100 c.f.u.) and treated with Ag85B-Z-DC on −1 and 4 dpi. RNA-seq was performed on lungs at 8 dpi. (a) Expression values of each biological replicate (n=5 for Vac, n=4 for Vac+Ag85B-Z-DC) for each group of a subset of selected genes of interest (all selected genes had a differential expression of at least twofold). These genes were differentially expressed using CuffDiff output with a P<0.05, and Benjamini–Hochberg false discovery rate (FDR) of 5%. The scaled expression of each replicate, denoted as the row Z-score, is plotted in red–blue colour scale with red indicating high expression and blue indicating low expression. q values for each gene of interest are shown in Supplementary Table 1 for the top 100 upregulated genes. (b) Expression of the top 100 upregulated genes shown in Supplementary Table 1 was considered in the published transcriptional data following expression in lungs of mice infected (red curves) with Mtb, or uninfected controls (blue curves) along the timecourse of 14, 21, 28 and 42 dpi33. (c) Gene Set Enrichment Analysis was used to analyse the expression of the top 100 upregulated genes shown in Supplementary Table 1 in the context of the infection model of Cd40l−/− mice with P. murina. Genes differentially expressed between wild type (WT) and Cd40l−/− mice on 32 days post-P. murina infection were compared and the top 1,000 expressed genes shown in the heat map, ranked by the level of differential expression between the two genotypes70. Orange lines indicate positions of 100 genes from Supplementary Table 1, P<10−3. (d) Numbers of CD11c+CD103+ cells in lungs of Mtb-infected-vaccinated Z-DC-recipient mice was determined using flow cytometry. n=4–5 biological replicates±s.d. ***P≤0.001 by Student’s t-test.
To identify pathways that were being induced in lungs of vaccinated mice receiving Z-DC transfer, we cross referenced the transcriptional signature of the vaccinated Z-DC-recipient Mtb-infected mice (Supplementary Table 1) against all publicly available RNASeq datasets. In this unbiased search, we found the significant regulation of immune pathways associated with early T-cell activation and APC function, present in our transcriptional data also coincided with expression in the lungs of wild-type mice infected with the pulmonary pathogen, Pneumocystis murina. Specifically, we found that several genes were induced both in the lungs of vaccinated Mtb-infected mice receiving Z-DC transfer, and following P. murina infection in wild-type mice. In contrast, these genes were not upregulated in Cd40l−/− mice infected with P. murina (Fig. 4c), suggesting CD40 axis dependence underlying the accelerated vaccine response in Z-DC transfer-recipient mice. Furthermore, Batf3 is a transcription factor that drives development of CD103+ DCs, which are important for priming Th1 and Th17 responses34,35. Batf3 messenger RNA expression was increased in vaccinated Mtb-infected hosts receiving Z-DC transfer (Supplementary Fig. 8) and coincided with increased accumulation of Batf3-dependent CD103+ DCs (Fig. 4d). Thus, we have identified a CD103+ DC and CD40 pathway that can potentially be targeted for improved Mtb control in vaccinated mice.
Activating CD103+ DCs improves Mtb control by vaccines
CD103+ DCs accumulate in vaccinated Mtb-infected hosts receiving DC transfer, and the CD40 pathway is activated in Z-DC transfer-induced protection against TB disease in vaccinated hosts. To target endogenous host mucosal DCs, we then assessed if activation of the CD40 pathway and targeting endogenous CD103+ mucosal DCs in standard BCG-vaccinated Mtb-infected mice, can mimic the effects of DC transfer and induce superior Mtb control. Amphiphilic-CpG (amph-CpG) in mice is a modified Toll-like receptor (TLR) 9 agonist that is efficiently taken up by DCs and macrophages, and enhances T-cell responses to peptide vaccines36. In addition, treatment of DCs with amph-CpG upregulates CD103 expression (Fig. 5a). FGK4.5 is an agonistic CD40 antibody that has been shown to activate DCs in murine cancer treatment models37,38. Thus, vaccinated Mtb-infected mice received amph-CpG along with FGK4.5, or either amph-CpG or FGK4.5 alone, delivered i.t. at −1 and 4 dpi, and early vaccine responses and Mtb burden in the lungs were assessed. We found that while delivery of either amph-CpG alone or FGK4.5 alone did not induce significant vaccine protection, delivery of amph-CpG and FGK4.5 together in vaccinated mice results in early Mtb control, similar to that seen in vaccinated Mtb-infected hosts receiving DC transfer (Fig. 5b). Importantly, delivery of amph-CpG and FGK4.5 induced accelerated activation of vaccine-induced cytokine-producing CD4+ T-cell responses (Fig. 5c–e) and coincided with activation of lung alveolar macrophages (Fig. 5f). Moreover, treatment with amph-CpG and FGK4.5 resulted in increased accumulation of CD103+ lung DCs in Mtb-infected-vaccinated mice (Fig. 5g; see gating strategy in Supplementary Fig. 4c). Thus, our data suggest that targeting host endogenous CD103+ DCs and activation of the CD40 pathway can overcome delayed antigen presentation and results in rapid activation of vaccine-induced T-cell responses and completely control Mtb growth.
(a) BMDC were cultured from B6 mice. After harvesting and resting overnight, BMDC were treated overnight with 1.24 nmol amph-CpG. CD103 expression on activated DCs was assessed by flow cytometry. (b–g) Mice were vaccinated with BCG s.c. and rested for 4 weeks. Vaccinated mice were infected with Mtb HN878 and treated with either Ag85B-Z-DC, amph-CpG (1.24 nmol), FGK4.5 (100 μg), or both amph-CpG and FGK4.5 along with Ag85B peptide (5 μg) on −1 and 4 dpi. Mice were collected at 8 dpi. (b) Lung bacterial burden was determined by plating. Flow cytometry was used to assess (c) numbers of CD4+CD44hi cells in the lungs, and (d) IL-17 and (e) IFN-γ production by CD4+ T cells, (f) MHC-II expression on alveolar macrophages and fold change MFI relative to Vac was calculated. (g) Flow cytometry was used to calculate numbers of CD11c+CD103+ cells in the lungs. (a) n=4–5 technical replicates±s.d., (b–g) n=4–10 biological replicates. *P≤0.05, **P≤0.01, ***P≤0.001 by Student’s t-test (a) or one-way analysis of variance (b–g). Dotted lines on bacterial burden plots represent the limit of detection by plating.
Discussion
Thus far, the development of sterilizing vaccines against TB for human use has not become a reality. This is especially concerning considering the status of TB, as one of the leading causes of death due to infectious diseases in the world today. In the current study, we hypothesized that our inability to develop a sterilizing vaccine against Mtb infection is not due to a failure of the vaccine to induce effective T-cell responses, but due to a delay in antigen presentation and subsequent delay in accumulation of vaccine-induced T-cell responses. Indeed, we show here that vaccine-induced T cells can respond to antigen exposure and induce mycobactericidal activity ex vivo. Transfer of exogenously primed DCs into lungs of vaccinated Mtb-infected mice can directly activate vaccine-induced T cells in vivo, leading to accelerated formation of protective B-cell follicles, rapid and early alveolar macrophage activation, and early control of establishment of Mtb infection. In addition, our results have identified novel mechanisms, involving CD103+ DCs and the CD40 activation pathway that may be therapeutically targeted to improve vaccine-induced protection during TB. Together, our data provide novel and mechanistic insights into pathways that can be targeted to generate sterilizing vaccine-induced immunity against TB.
Most modern TB vaccines induce lung and systemic Th1 and/or Th17 vaccine responses2. In animal models, however, none of these vaccines induce sterilizing immunity to TB, instead only decreasing the lung Mtb burden by 0.5–1.0 log (refs 3, 8, 9, 10, 11). Our studies show that vaccine-induced CD4+ Mtb-specific T cells isolated from the lung can undergo activation rapidly, proliferate and produce both IFN-γ and IL-17 ex vivo. Importantly, we show that proliferating vaccine-induced CD4+ T cells can activate macrophages to control Mtb, suggesting that there is no inherent deficiency in vaccine-induced CD4+ recall responses. In addition, lung-resident vaccine-induced CD4+ T-cell responses are long lasting39 and adoptive transfer of T cells isolated from Mtb-infected animals into newly infected animals confers protective immunity40,41,42,43,44. Mtb is a successful pathogen, known to inhibit MHC-II transactivator expression, MHC-II expression and antigen presentation in APCs18. Thus, the inability of TB vaccines to confer sterilizing immunity on Mtb challenge may be associated with delayed CD4+ T-cell responses due to delayed APC function, rather than just poor induction of vaccine-induced CD4+ T cells. Accordingly, targeting DCs in vaccinated hosts can substantially accelerate the activation and accumulation of cytokine-producing CD4+ T-cell recall responses in the lung, initiate rapid activation of alveolar macrophages and limit Mtb growth. Interestingly, pre-clinical vaccines that have come closest to inducing sterilizing immunity following Mtb infection have been recombinant mycobacterial vaccines delivered mucosally15,45. BCG is known to reduce the overall childhood morbidity caused by infections other than Mtb46, which could be due to the ability of BCG to induce ‘innate memory’ by epigenetically altering the phenotype of infected cells to one producing increased inflammatory cytokines, via autophagy and nucleotide-binding oligomerization domain-containing protein 2 (NOD2)-dependent pathways47,48. Thus, it is possible that recombinant mycobacterial vaccines delivered mucosally target pathways similar to DC transfer through activation of lung-resident DCs and macrophages, thereby driving earlier activation of lung-resident T cells on Mtb challenge. Further work delineating the specific mechanisms by which vaccine-induced immunity can be accelerated by targeting these pathways will therefore be highly relevant.
Previous studies have attempted to improve DC function at the time of vaccination by targeting the CD40 activation pathway49, DC receptor Dec-205 (refs 50, 51), or by utilizing cell-derived vaccines, such as Mtb antigen-primed DCs31,32. Improving DC function by targeting the CD40 pathway during vaccination had no effect49, while activating Dec-205 at the time of vaccination had a small effect (∼0.5 log reduction) on vaccine-induced protection on Mtb challenge50,51. Vaccination with antigen-primed DCs has generated mixed results in Mtb-infected mice, with a study demonstrating a negative impact of DC vaccination due to exuberant inflammation31, or induction of vaccine-induced protection similar to levels seen in BCG-vaccinated hosts32. Our results show that use of Z-DCs as a mucosal vaccination tool as boost to BCG vaccination has a small protective effect on Mtb infection, but not as effective as use of Z-DC transfer at the time of infection. In contrast, only very few studies have therapeutically manipulated early innate responses following Mtb infection. Delivery of polyI:C, a TLR3 ligand, exacerbated inflammation and increased Mtb burden52. In contrast, delivery of FimH, a TLR4 ligand, improved T-cell responses and resulted in ∼1 log reduction in lung bacterial burden, following high-dose intranasal infection with Mtb H37Ra (ref. 53). To our knowledge, no published studies have manipulated early host responses in vaccinated Mtb-infected hosts, with resulting complete early Mtb control. Furthermore, both CpG (reviewed in refs 54, 55) and CD40 agonists (reviewed in ref. 56) have been safely tested in clinical human trials, predominantly for cancer treatment. Thus, repurposing these host-directed therapeutics, as well as other activators of CD103+ DC function57,58, for rapid activation of vaccine-induced T-cell responses may thus be useful in development of novel drugs or vaccination approaches for TB.
Our results show that while delivery of unstimulated DCs by itself improves vaccine-induced CD4+ T-cell responses and Mtb control in vaccinated hosts, zymosan activation of DCs, especially generated from Il10−/− mice confers superior vaccine-induced Mtb control. These results support the idea that delayed antigen presentation and subsequent delayed induction of vaccine-induced T-cell activation is a major bottleneck for TB vaccine efficacy. In addition, these results support an important role for IL-10 in limiting TB vaccine efficacy and early Mtb control in vaccinated hosts28,29. Furthermore, Z-DC transfer works in both standard BCG vaccinated as well as in mucosally vaccinated Mtb-infected hosts, the route of vaccination may define the mechanisms of protection. In mucosally vaccinated Mtb-infected mice receiving DC transfer, mucosal lung-resident CD4+ T cells, primarily IL-17-producing cells are activated and accumulate rapidly in the lung, thus mediating the superior protection associated with this model. In BCG-vaccinated Mtb-infected mice receiving DC transfer, DC transfer can still prime a population of lung-resident IL-17-producing vaccine-induced CD4+ T cells, but likely DC migration to LNs is required to mobilize IFN-γ-producing vaccine-induced CD4+ T-cell responses from the systemic pool, to provide enhanced Mtb control. Regardless, that we can target CD103+ DCs and the CD40 activation pathway to limit Mtb growth in standard BCG-vaccinated Mtb-infected hosts suggest that our strategy is effective in overcoming a bottleneck associated with delayed induction of vaccine-induced CD4+ T-cell responses in vaccinated hosts. This is further supported by our results showing that DC transfer can protect vaccinated hosts on challenge with Mtb strains belonging to different lineages, suggesting that T-cell bottleneck for TB vaccines is universal. In addition, that Z-DC transfer into unvaccinated hosts can also accelerate naive T-cell priming and improve primary immunity, albeit not to the effectiveness seen in vaccinated hosts, suggest the DC transfer may be useful to enhance both primary and vaccine-induced pathways during Mtb infection.
DC transfer not only induces the rapid activation of vaccine-induced CD4+ T-cell responses in vaccinated Mtb-infected mice, but also has substantial downstream effects on activation of myeloid pathways in the lung. First, lung alveolar macrophages are rapidly activated in vaccinated hosts by 8 dpi, an event that does not happen until 14 dpi in vaccinated hosts and until 21 dpi in unvaccinated hosts3. Accordingly, the early global transcriptional changes in the lungs of vaccinated Mtb-infected mice receiving DC transfer mirror the transcriptional changes occurring in 21 dpi Mtb-infected lungs33. Furthermore, we observe early accumulation of myeloid DCs, including CD103+ DCs and an enhancement of DC activation pathways, in particular genes associated with CD40 activation in the lungs of vaccinated Mtb-infected mice. CD103+ DCs are a tissue-resident DC subset, which have primarily been implicated in cross-presentation and induction of CD8+ T-cell responses59,60. More recently, however, CD103+ DCs have been shown to induce CD4+ T cells34,35,61. In the lung, CD103+ DCs represent a migratory DC subset, with the ability to migrate from the lungs to the LN and back again62, and induce both Th1 and Th17 cytokines by CD4+ T cells34,35,63. Although antigen presentation by transferred DCs likely directly activates vaccine-induced CD4+ T-cell responses, rapid activation of CD4+ T-cell responses also modifies the lung micro-environment to improve subsequent antigen presentation by endogenous host APCs. We show here that targeting DC activation pathways through the TLR ligand amph-CpG, along with use of the CD40 agonist FGK4.5, similarly control Mtb growth. Treatment of DCs with granulocyte–macrophage-colony-stimulating factor (GM-CSF), a by-product of TLR9 stimulation through CpG64 has previously been shown to upregulate CD103 expression65. Thus, it is possible that CpG stimulates CD103+ expression on DCs indirectly through inducing GM-CSF by other lung-resident cells. Upregulation of CD103 as well as recruitment of CD103+ DCs to the lung alone, however, are not sufficient for improved T-cell activation, suggesting that the activating signal delivered through the CD40 agonist FGK4.5 provides the second signal required for improved early antigen presentation to vaccine-induced CD4+ T cells. Therefore, Mtb-infected individuals likely have a population of antigen-responsive T cells, and targeting DCs as a potential therapy, may provide the lung-resident T cells the necessary signals to proliferate and control Mtb infection.
Thus, our data for the first time show that delayed activation of vaccine-induced T-cell responses is a critical bottleneck for TB vaccine efficacy. Our results demonstrate that this delay can be overcome by DC transfer, or by targeting DC activation, leading to rapid T-cell recall responses and enhanced alveolar macrophage activation, resulting in complete control of early Mtb growth. These results provide a roadmap for the type of immune responses that a sterilizing TB vaccine should induce, representing an important milestone in our mechanistic understanding of TB vaccine-mediated immune responses. Our results suggest that despite induction of effective Mtb-specific vaccine T-cell responses by modern TB vaccines66,67,68, delayed T-cell vaccine responses on Mtb infection may pose a significant hurdle to development of a sterilizing vaccine for TB in humans. Therefore, it is possible that the development of a sterilizing vaccine for TB may not be a realistic goal. Instead, redirecting our efforts towards developing novel vaccine strategies and therapeutics for preventing the reactivation of latent TB and TB transmission may benefit global control of TB.
Methods
Mice
C57BL/6 (B6), B6.129P2-Il10tm1Cgn/J (Il10−/−) and B6.129 (Cg)-Foxp3tm3(DTR/GFP)Ayr/J (Foxp3.DTR) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and bred at Washington University in St Louis. C57BL/6-Tg(H2-Kb-Tcra,-Tcrb)P25Ktk/J (P25 TCR Tg) and B6.PL-Thy1a/CyJ (Thy1.1) mice were purchased from Jackson Laboratories, and crossed and bred at Washington University in St Louis. Mice (male and female) were used at 6–8 weeks of age. Experiments were designed according to empirical statistical power analysis. All mice were used and housed in accordance with the National Institute of Health guidelines for housing and care of laboratory animals, and permission for the experiments in this study was granted by the Washington University in St Louis Institutional Animal Care and Use Committee under protocol 20130195. All efforts were made to minimize pain and suffering as described in this protocol.
Bacterial infection and vaccination
M. bovis Bacille Calmette–Guerin (BCG Pasteur, Source: Trudeau institute), Mtb strain HN878 (Source: BEI Resources) and Mtb strain H37Rv (Source: Trudeau Institute) were grown to mid-log phase in Proskauer Beck medium containing 0.05% Tween80 and frozen in 1 ml aliquots at −80 °C.
Mice were vaccinated with 1 × 106 c.f.u. BCG s.c.4 and 4 weeks later received 133 μg Ag85B240–254 peptide (New England Peptide, Gardner, MA, USA) along with 1 μg heat-labile enterotoxin (LT-IIb) intranasally in 20 μl (10 μl per nare). In some experiments, mice only received mucosal vaccination with three doses, 2 weeks apart of 133 μg Ag85B240–254 peptide in 1 μg LT-IIb intranasally. In other experiments, mice only received 1 × 106 c.f.u. BCG s.c. as a model of parenteral vaccination. Four or 10 weeks after the final vaccination, mice were infected with 100 c.f.u. (low dose) or 1,000 c.f.u. (high dose) Mtb HN878 via aerosol using a Glas-Col aerosol exposure system (Glas-Col, Terre Haute, IN, USA)4. At given time points following infection, organs were collected, homogenized and tissue homogenates plated in serial dilutions on 7H11 agar (BD Biosciences, San Jose, CA, USA) to assess bacterial burden4.
In experiments using Foxp3.DTR mice, mice were treated with three doses 2 days apart of 10 μg kg−1 diphtheria toxin (Millipore, Billerica, MA, USA)69 for depletion of Foxp3+ cells, starting 8 dpi.
In vitro culture of DCs and macrophages
BMDC and bone marrow-derived macrophages (BMDMs) were generated from B6 or Il10−/− mice. Cells were isolated from the femur and tibia, and cultured at 1 × 106 cells ml−1 in 10 ml complete DMEM (cDMEM) supplemented with 20 ng ml−1 recombinant mouse GM-CSF (Peprotech, Rocky Hill, NJ, USA) for 3 days at 37 °C in 7.5% CO2, at which point, an additional 10 ml cDMEM supplemented with 20 ng ml−1 recombinant mouse GM-CSF was added. Non-adherent cells (BMDCs) were collected on the seventh day of culture, and counted and plated at 2 × 106 cells ml−1 in cDMEM. Adherent BMDMs were collected at the same time by scraping and cultured at 1 × 106 cells ml−1. For BMDC stimulation, Ag85B240–254 peptide (20 μg ml−1) and Zymosan (Invivogen, San Diego, CA) was added at 25 μg ml−1. Cells were stimulated for 16 h before being collected, washed, counted and instilled i.t. into mice at 1 × 106 cells in 50 μl on the day before infection and 4 dpi. Supernatants from stimulated in vitro cultures were collected and frozen at −80 °C for analysis by enzyme-linked immunosorbent assay and multiplex assay. In some experiments, BMDCs were treated with 1.24 nmol amph-CpG.
Amph-CpG and FGK4.5 treatment of mice
Amph-CpG was prepared as described previously36. Briefly, solid phase DNA synthesis and 5′ lipophilic conjugation were carried out using an ABI 394 synthesizer36. The sequence used was murine oligodeoxynucleotides (ODN) class B sequence 1,826 with two guanine spacers: 5′-diacyl lipid-*G*G*T*C*C*A*T*G*A*C*G*T*T*C*C*T*G*A*C*G*T*T-3′. Amph-CpG was delivered to mice in 50 μl i.t. at 1.24 nmol per mouse. The CD40 agonist FGK4.5 (R&D Systems, Minneapolis, MN, USA) was delivered in 50 μl i.t. at 100 μg per mouse. Both treatments were delivered with 5 μg Ag85B peptide.
Antibody treatment of mice
In some experiments, mice were treated with CD4 (clone GK1.5, BioXcell) depleting antibodies delivered intraperitoneally, starting on the day of Mtb infection and 7 days later (300 μg per mouse).
Generation of single-cell suspensions from tissues
Lung single-cell suspensions from vaccinated or Mtb-infected mice were generated as before4. For LNs and spleens, organs were passed through a 70 μm cell strainer and then processed as for lungs4. For flow cytometric analysis, cells were either stained immediately, or stimulated for 18 h in the presence of Ag85B240–254 peptide, with GolgiStop (5 μl ml−1; BD Biosciences) and Brefeldin A (1 μg ml−1; BioLegend, San Diego, CA, USA) added for the last 5 h. Intracellular cytokine staining was performed using the BD Cytofix/Cytoperm kit (BD Biosciences). Cells were collected using a BD FACS Jazz or a BD LSR Fortessa, and analysis was performed using FlowJo (Treestar, Ashland, OR, USA). Antibodies used include anti-mouse CD11c (clone HL3; BD Biosciences; dilution: 1/200), anti-mouse MHC-II (clone M5/114.15.2; Tonbo Biosciences, San Diego, CA, USA; dilution: 1/150), anti-mouse CD3 (clone 500A2; BD Biosciences; dilution: 1/200), anti-mouse CD4 (clone RM4-5; BD Biosciences, dilution: 1/200), anti-mouse CD44 (clone IM7; eBioscience, dilution: 1/400), anti-mouse IL-17 (clone TC11-18H10; BD Biosciences, dilution: 1/100), anti-mouse IFN-γ (XMG1.2; BD Biosciences; dilution: 1/100), anti-mouse Thy1.1 (clone OX-7; BD Biosciences; dilution: 1/100) and anti-mouse CD103 (clone 2E7, eBioscience; dilution: 1/200).
ELISpot
For analysis of production of antigen-specific cytokines by enzyme-linked immunospot (ELISpot) assay as before4, 96-well ELISpot plates were coated with monoclonal anti-mouse IL-17 antibody (clone 50101.111; R&D systems, Minneapolis, MN, USA) or anti-mouse IFN-γ antibody (clone R4-6A2; eBioscience) overnight, then blocked with media containing 10% fetal bovine serum. Total cells from lungs, or 5 × 105 cells from LN were plated in the top well and subsequently diluted twofold in serial dilutions. Irradiated B6 splenocytes were used as APCs at a concentration of 1 × 106 cells per well in the presence of 10 μg ml−1 peptide and 10 U ml−1 IL-2. After 24 h, plates were washed and probed with biotinylated anti-mouse IL-17 antibody (clone eBio17B7; eBioscience) or biotinylated anti-mouse IFN-γ antibody (clone XMG1.2; eBioscience), and binding detected using streptavidin–alkaline phosphatase (Vector Laboratories, Burlingame, CA, USA) with BCIP/NBT (Sigma Aldrich) as the substrate. Spots were visualized and enumerated using a CTL-Immunospot S5 MicroAnalyzer (C.T.L., Shaker Heights, OH, USA).
Cell proliferation and mycobacterial killing assays
CD4+ T cells were isolated from single-cell suspensions generated from LNs and spleen from Ag85B TCR Tg mice on a Thy1.1 background using a CD4 L3T4 positive selection kit according to the manufacturer’s instructions (Miltenyi Biotec Inc, San Diego, CA, USA). Naive CD4+ T cells (Th0) were intravenously transferred into B6 mice (on a Thy1.2 background) at 2 × 106 cells per mouse. For assessment of proliferation ex vivo, whole lung cells isolated from mice receiving adoptively transferred T cells were labelled with 1 μM carboxyfluorescein succyinimidyl ester (CFSE, Invitrogen, Thermo Fisher) for 15 min at 37 °C at 1 × 107 cells ml−1 in incomplete DMEM3. Cells were then cultured for 3 or 6 days with Il10−/− BMDC stimulated overnight with zymosan alone (25 μg ml−1) or zymosan (25 μg ml−1)+Ag85B240–254 peptide (20 μg ml−1) in the presence or absence of Mtb HN878. Supernatants were collected and frozen at −80 °C. Cells were collected and T-cell proliferation was assessed by measuring carboxyfluorescein succyinimidyl ester dilution by flow cytometry. Transferred antigen-specific cells were tracked in vivo using Thy1.1 staining. Cell activation was measured by CD44 staining.
For the mycobacterial killing assay, BMDMs were infected overnight with Mtb HN878 at an multiplicity of infection of 1 in antibiotic-free cDMEM. The following day, infected BMDMs were co-cultured for 6 days at a 1:1 ratio with T cells previously activated with Ag85B-Z-DC as described above. Intracellular Mtb c.f.u. was assessed by lysing the BMDMs with 0.05% SDS before plating on 7H11 plates in serial dilutions.
Cytokine and chemokine production
Cytokine and chemokine production in supernatants collected from stimulated BMDC or from T-cell-BMDC cultures was analysed using Milliplex Multiplex Assays (Millipore), using the protocol specified by the manufacturer. IL-10 (R&D; cat. no: DY417), IL-23 (R&D; cat. no: DY1887) and IL-6 (R&D; cat. no: DY406) were measured using a Duoset kit (R&D Systems), using the protocol specified by the manufacturer. IL-1β (BD; cat. no: 559603) was measured using an BD OptEIA IL-1β ELISA Set (BD Biosciences) using the protocol specified by the manufacturer.
Immunofluorescence staining
Lung lobes were perfused with 10% formalin, fixed and paraffin embedded. For immunofluorescent staining, lung sections were cut, immersed in xylene to remove paraffin and then hydrated in 96% alcohol and phosphate-buffered saline. Antigens were unmasked with a DakoCytomation Target Retrieval Solution (Dako, Carpinteria, CA, USA), and non-specific binding was blocked with 5% (v/v) normal donkey serum and Fc block (BD Pharmingen). Endogenous biotin was neutralized by adding avidin, followed by incubation with biotin (Sigma Aldrich). Sections were probed with anti-B220 to detect B cells (clone RA3-6B2, BD Pharmingen; dilution: 1/100) and anti-CD3 to detect T cells (clone M-20, Santa Cruz Biotechnology, Santa Cruz, CA; dilution: 1/100). For analysis of B-cell follicles, follicles were outlined with the automated tool of the Zeiss Axioplan 2 microscope (Zeiss, Thornwood, NY, USA), and total area and average size was calculated in squared microns.
Quantitative PCR with reverse transcription
Total RNA was isolated from lung tissue using an RNeasy RNA isolation kit (Qiagen, Valencia, CA, USA). Complementary DNA was generated using ABI reverse transcription reagents (ABI, Thermo Fisher) on a BioRad DNA Engine Thermal Cycler (BioRad, Hercules, CA, USA). Gene expression was assessed using primers from integrated DNA technologies (IDT) (iNOS (F-CAGCTGGGCTGTACAAACCTTC; R-CATTGGAAGTGAAGCGTTTCG;PROBE5′-/56-FAM/CGG GCA GCC TGT GAG ACC TTT GA/3BHQ_1/-3′);GAPDH (F-CTCGTCCCGTAGACAAAATGG; R-AATCTCCACTTTGCCACTGCA; PROBE5′-/56-FAM/CGG ATT TGG CCG TAT TGG GCG/3BHQ_1/-3′)) (Coralville, IA, USA) and ABI (CCL19: Mm00839967_g1; Saa3: Mm00441203-ml; Lcn2: Mm01324472_g1;Batf3: Mm01318274_m1; Itgae: Mm00434443_m1; Col1a1: Mm00801666_g1) and run on a Viia7 Real-Time PCR system (Life Technologies, Thermo Fisher). Expression of genes of interest was normalized to GAPDH expression, and log 10-fold induction over the control group was assessed using the ΔΔCT calculation.
RNA-seq and gene set enrichment analysis
Total RNA was isolated from lung tissue using an RNeasy RNA isolation kit (Qiagen, Valencia, CA, USA). Each sample was assessed using Qubit 2.0 fluorometer (Invitrogen, Thermo Fisher) and Agilent Tapestation 2200 (Agilent Technologies, Santa Clara, CA, USA). Sequencing libraries were generated using Illumina TruSeq RNA Access library prep kit (Illumina, San Diego, CA, USA) following the manufacturer’s protocol. Cluster generation and 75 bp single read single-indexed sequencing was performed on Illumina NextSeq 500 (Illumina). Sequencing analysis was done using mRNA-seq Analysis on Maverix Analytic Platform (Maverix Biomics, Inc, San Mateo, CA). Raw sequencing reads from Illumina sequencing platform that was converted into FASTQ file format were quality checked for potential sequencing issues and contaminants using FastQC. Adapter sequences, primers, Ns and reads with quality score <28 were trimmed using fastq-mcf of ea-utils and Trimmomatic. Reads with a remaining length of fewer than 20 bp after trimming were discarded. Single reads were mapped to the mouse genome (m10) using STAR in a strand specific manner. Cufflinks was used to determine fragments per kilobase of transcript per million mapped reads (FPKM) levels for each gene from the STAR alignment and was used as input for Cuffdiff. Pairwise differential expression was quantified using Cuffdiff. Read counts were then normalized across all samples and significant differentially expressed genes were determined by adjusted P value with a threshold of 0.05. For Gene Set Enrichment Analysis we have selected expressed genes from GSE11005 (top 10,000 based on average expression level). We then assembled ranked list using signed statistics values and performed pre-ranked GSEA using top 100 vaccine upregulated genes as a query gene set. The data shown is uncorrected for multiple comparisons. We also performed differential expression analysis using Limma between wild type and Cd40l−/− groups at day 32 post-P. murina infection.
Statistical analysis
Statistical analyses were performed using GraphPad Prism (La Jolla, CA, USA). For experiments with two groups, two-tailed Student’s t-tests or Mann–Whitney test were performed. For two or more groups, a one-way analysis of variance or Kruskall–Wallis test was used. For multiple groups with multiple time points, a two-way analysis of variance was used. All plots show mean±s.d.
Data availability
Sequence data that support the findings of this study have been deposited in BioProject with the primary accession code SRP091809. Other data that support the findings of this study are available from the corresponding author upon request.
Additional information
How to cite this article: Griffiths, K. L. et al. Targeting dendritic cells to accelerate T-cell activation overcomes a bottleneck in tuberculosis vaccine efficacy. Nat. Commun. 7, 13894 doi: 10.1038/ncomms13894 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Dye, C. & Williams, B. G. The population dynamics and control of tuberculosis. Science 328, 856–861 (2010).
Griffiths, K. L. & Khader, S. A. Novel vaccine approaches for protection against intracellular pathogens. Curr. Opin. Immunol. 28, 58–63 (2014).
Khader, S. A. et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8, 369–377 (2007).
Gopal, R. et al. Interleukin-17-dependent CXCL13 mediates mucosal vaccine-induced immunity against tuberculosis. Mucosal Immunol. 6, 972–984 (2013).
Aguilo, N. et al. Pulmonary but Not Subcutaneous Delivery of BCG Vaccine Confers Protection to Tuberculosis-Susceptible Mice by an Interleukin 17-Dependent Mechanism. J. Infect. Dis. 213, 831–839 (2015).
Monin, L. et al. Immune requirements for protective Th17 recall responses to Mycobacterium tuberculosis challenge. Mucosal Immunol. 8, 1099–1109 (2015).
Desel, C. et al. Recombinant BCG DeltaureC hly+ induces superior protection over parental BCG by stimulating a balanced combination of type 1 and type 17 cytokine responses. J. Infect. Dis. 204, 1573–1584 (2011).
Aagaard, C. et al. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat. Med. 17, 189–194 (2011).
Bertholet, S. et al. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci. Transl. Med. 2, 53ra74 (2010).
Goonetilleke, N. P. et al. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J. Immunol. 171, 1602–1609 (2003).
Tchilian, E. Z. et al. Immunogenicity and protective efficacy of prime-boost regimens with recombinant (delta)ureC hly+ Mycobacterium bovis BCG and modified vaccinia virus ankara expressing M. tuberculosis antigen 85A against murine tuberculosis. Infect. Immun. 77, 622–631 (2009).
Sweeney, K. A. et al. A recombinant Mycobacterium smegmatis induces potent bactericidal immunity against Mycobacterium tuberculosis. Nat. Med. 17, 1261–1268 (2011).
Grode, L. et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J. Clin. Invest. 115, 2472–2479 (2005).
Martin, C. et al. The live Mycobacterium tuberculosis phoP mutant strain is more attenuated than BCG and confers protective immunity against tuberculosis in mice and guinea pigs. Vaccine 24, 3408–3419 (2006).
Kaushal, D. et al. Mucosal vaccination with attenuated Mycobacterium tuberculosis induces strong central memory responses and protects against tuberculosis. Nat. Commun. 6, 8533 (2015).
Reiley, W. W. et al. ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes. Proc. Natl Acad. Sci. USA 105, 10961–10966 (2008).
Wolf, A. J. et al. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J. Exp. Med. 205, 105–115 (2008).
Harding, C. V. & Boom, W. H. Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nat. Rev. Microbiol. 8, 296–307 (2010).
Urdahl, K. B. Understanding and overcoming the barriers to T cell-mediated immunity against tuberculosis. Semin. Immunol. 26, 578–587 (2014).
Srivastava, S., Grace, P. S. & Ernst, J. D. Antigen export reduces antigen presentation and limits T cell control of M. tuberculosis. Cell Host Microbe 19, 44–54 (2016).
Jung, Y. J., Ryan, L., LaCourse, R. & North, R. J. Properties and protective value of the secondary versus primary T helper type 1 response to airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 201, 1915–1924 (2005).
Srivastava, S. & Ernst, J. D. Cell-to-cell transfer of M. tuberculosis antigens optimizes CD4 T cell priming. Cell Host Microbe 15, 741–752 (2014).
Vilaplana, C. et al. To achieve an earlier IFN-gamma response is not sufficient to control Mycobacterium tuberculosis infection in mice. PLoS ONE 9, e100830 (2014).
Shafiani, S., Tucker-Heard, G., Kariyone, A., Takatsu, K. & Urdahl, K. B. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J. Exp. Med. 207, 1409–1420 (2010).
Quinn, K. M. et al. Inactivation of CD4+ CD25+ regulatory T cells during early mycobacterial infection increases cytokine production but does not affect pathogen load. Immunol. Cell Biol. 84, 467–474 (2006).
Manca, C. et al. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha /beta. Proc. Natl Acad. Sci. USA 98, 5752–5757 (2001).
Gerosa, F. et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J. Exp. Med. 205, 1447–1461 (2008).
Gopal, R. et al. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur. J. Immunol. 42, 364–373 (2012).
Pitt, J. M. et al. Blockade of IL-10 signaling during bacillus Calmette-Guerin vaccination enhances and sustains Th1, Th17, and innate lymphoid IFN-gamma and IL-17 responses and increases protection to Mycobacterium tuberculosis infection. J. Immunol. 189, 4079–4087 (2012).
Ordway, D. J. et al. Mycobacterium bovis BCG-Mediated Protection against W-Beijing Strains of Mycobacterium tuberculosis Is Diminished Concomitant with the Emergence of Regulatory T Cells. Clin. Vaccine Immunol. 18, 1527–1535 (2011).
Gonzalez-Juarrero, M., Turner, J., Basaraba, R. J., Belisle, J. T. & Orme, I. M. Florid pulmonary inflammatory responses in mice vaccinated with Antigen-85 pulsed dendritic cells and challenged by aerosol with Mycobacterium tuberculosis. Cell. Immunol. 220, 13–19 (2002).
McShane, H., Behboudi, S., Goonetilleke, N., Brookes, R. & Hill, A. V. Protective immunity against Mycobacterium tuberculosis induced by dendritic cells pulsed with both CD8(+)- and CD4(+)-T-cell epitopes from antigen 85A. Infect. Immun. 70, 1623–1626 (2002).
Koo, M. S. et al. Phosphodiesterase 4 inhibition reduces innate immunity and improves isoniazid clearance of Mycobacterium tuberculosis in the lungs of infected mice. PLoS ONE 6, e17091 (2011).
Sergio, C. A., Bertolini, T. B., Gembre, A. F., Prado, R. Q. & Bonato, V. L. CD11c(+) CD103(+) cells of Mycobacterium tuberculosis-infected C57BL/6 but not of BALB/c mice induce a high frequency of interferon-gamma- or interleukin-17-producing CD4(+) cells. Immunology 144, 574–586 (2015).
Zelante, T. et al. CD103(+) Dendritic Cells Control Th17 Cell Function in the Lung. Cell Rep. 12, 1789–1801 (2015).
Liu, H. et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature 507, 519–522 (2014).
Kayes, T. et al. Agonistic anti-CD40 induces thyrocyte proliferation and promotes thyroid autoimmunity by increasing CD40 expression on thyroid epithelial cells. J. Immunol. 190, 3928–3938 (2013).
Zippelius, A., Schreiner, J., Herzig, P. & Muller, P. Induced PD-L1 expression mediates acquired resistance to agonistic anti-CD40 treatment. Cancer Immunol. Res. 3, 236–244 (2015).
Lindenstrom, T. et al. Vaccine-induced th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset. Infect. Immun. 80, 3533–3544 (2012).
Andersen, P. & Smedegaard, B. CD4(+) T-cell subsets that mediate immunological memory to Mycobacterium tuberculosis infection in mice. Infect. Immun. 68, 621–629 (2000).
Feng, C. G. & Britton, W. J. CD4+ and CD8+ T cells mediate adoptive immunity to aerosol infection of Mycobacterium bovis bacillus Calmette-Guerin. J. Infect. Dis. 181, 1846–1849 (2000).
Kipnis, A., Irwin, S., Izzo, A. A., Basaraba, R. J. & Orme, I. M. Memory T lymphocytes generated by Mycobacterium bovis BCG vaccination reside within a CD4 CD44lo CD62 Ligandhi population. Infect. Immun. 73, 7759–7764 (2005).
Mainali, E. S. & McMurray, D. N. Protein deficiency induces alterations in the distribution of T-cell subsets in experimental pulmonary tuberculosis. Infect. Immun. 66, 927–931 (1998).
Sakai, S. et al. Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. J. Immunol. 192, 2965–2969 (2014).
Marcus, S. A., Steinberg, H. & Talaat, A. M. Protection by novel vaccine candidates, Mycobacterium tuberculosis DeltamosR and DeltaechA7, against challenge with a Mycobacterium tuberculosis Beijing strain. Vaccine 33, 5633–5639 (2015).
Garly, M. L. et al. BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa. A non-specific beneficial effect of BCG? Vaccine 21, 2782–2790 (2003).
Buffen, K. et al. Autophagy controls BCG-induced trained immunity and the response to intravesical BCG therapy for bladder cancer. PLoS Pathog. 10, e1004485 (2014).
Kleinnijenhuis, J. et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl Acad. Sci. USA 109, 17537–17542 (2012).
Demangel, C. et al. Stimulation of dendritic cells via CD40 enhances immune responses to Mycobacterium tuberculosis infection. Infect. Immun. 69, 2456–2461 (2001).
Stylianou, E. et al. Exploring the vaccine potential of Dec-205 targeting in Mycobacterium tuberculosis infection in mice. Vaccine 29, 2279–2286 (2011).
Silva-Sanchez, A. et al. ESAT-6 targeting to DEC205+ antigen presenting cells induces specific-T cell responses against ESAT-6 and reduces pulmonary infection with virulent Mycobacterium tuberculosis. PLoS ONE 10, e0124828 (2015).
Antonelli, L. R. et al. Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J. Clin. Invest. 120, 1674–1682 (2010).
Lai, R. et al. Restoration of innate immune activation accelerates Th1-cell priming and protection following pulmonary mycobacterial infection. Eur. J. Immunol. 44, 1375–1386 (2014).
Bode, C., Zhao, G., Steinhagen, F., Kinjo, T. & Klinman, D. M. CpG DNA as a vaccine adjuvant. Expert. Rev. Vaccines 10, 499–511 (2011).
Zent, C. S. et al. Phase I clinical trial of CpG oligonucleotide 7909 (PF-03512676) in patients with previously treated chronic lymphocytic leukemia. Leuk. Lymphoma 53, 211–217 (2012).
Vonderheide, R. H. & Glennie, M. J. Agonistic CD40 antibodies and cancer therapy. Clin. Cancer Res. 19, 1035–1043 (2013).
Beauchamp, N. M., Yammani, R. D. & Alexander-Miller, M. A. CD8 marks a subpopulation of lung-derived dendritic cells with differential responsiveness to viral infection and toll-like receptor stimulation. J. Virol. 86, 10640–10650 (2012).
Semmrich, M. et al. Directed antigen targeting in vivo identifies a role for CD103+ dendritic cells in both tolerogenic and immunogenic T-cell responses. Mucosal Immunol. 5, 150–160 (2012).
Fuertes, M. B. et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J. Exp. Med. 208, 2005–2016 (2011).
Hildner, K. et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 322, 1097–1100 (2008).
Ashok, D. et al. Cross-presenting dendritic cells are required for control of Leishmania major infection. Eur. J. Immunol. 44, 1422–1432 (2014).
del Rio, M. L., Rodriguez-Barbosa, J. I., Kremmer, E. & Forster, R. CD103- and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J. Immunol. 178, 6861–6866 (2007).
Furuhashi, K. et al. Mouse lung CD103+ and CD11bhigh dendritic cells preferentially induce distinct CD4+ T-cell responses. Am. J. Respir. Cell Mol. Biol. 46, 165–172 (2012).
Souza-Fonseca-Guimaraes, F. et al. Interferon-gamma and granulocyte/monocyte colony-stimulating factor production by natural killer cells involves different signaling pathways and the adaptor stimulator of interferon genes (STING). J. Biol. Chem. 288, 10715–10721 (2013).
Zhan, Y. et al. GM-CSF increases cross-presentation and CD103 expression by mouse CD8(+) spleen dendritic cells. Eur. J. Immunol. 41, 2585–2595 (2011).
Santosuosso, M., McCormick, S., Zhang, X., Zganiacz, A. & Xing, Z. Intranasal boosting with an adenovirus-vectored vaccine markedly enhances protection by parenteral Mycobacterium bovis BCG immunization against pulmonary tuberculosis. Infect. Immun. 74, 4634–4643 (2006).
Skeiky, Y. A. et al. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J. Immunol. 172, 7618–7628 (2004).
Hoang, T. et al. ESAT-6 (EsxA) and TB10.4 (EsxH) based vaccines for pre- and post-exposure tuberculosis vaccination. PLoS ONE 8, e80579 (2013).
Boehm, F. et al. Deletion of Foxp3+ regulatory T cells in genetically targeted mice supports development of intestinal inflammation. BMC Gastroenterol. 12, 97 (2012).
Hernandez-Novoa, B. et al. Immune responses to Pneumocystis murina are robust in healthy mice but largely absent in CD40 ligand-deficient mice. J. Leukoc. Biol. 84, 420–430 (2008).
Acknowledgements
This work was supported by Washington University in St Louis, National Institute of Health grant HL105427, AI127172 to S.A.K., by the Ragon Institute of MGH, MIT, and Harvard and the U.S. Army Research Office through the Institute for Soldier Nanotechnologies (contract W911NF-13-D-0001) to D.J.I., American Lung Association Senior Research Training Fellowship RT-30592, Department of Molecular Microbiology, Washington University St Louis, and Alexander and Gertrude Berg Fellowship to K.L.G. J.R.-M. was supported by funds of the Department of Medicine, University of Rochester, and U19 AI91036. We thank Sarah Squires for animal breeding, and Drs June Treerat, Racquel Domingo-Gonzalez for technical assistance. D.J.I. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
K.L.G., M.A., R.G., J.K.K. and S.A.K. designed the experiments; K.L.G., M.A., S.D., K.D.M., R.G., W.H. and J.R-M. performed the experiments; K.L.G., W.H., T.D.C., J.K.K., J.R-M., M.N.A. and S.A.K. performed analyses; T.D.C., D.J.I provided reagents; K.L.G., S.D. and S.A.K. wrote the paper; K.L.G., T.D.C., D.J.I., J.K.K., J.R-M. and S.A.K. edited the paper. S.A.K., D.J.I., J.R-M. provided funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures and Supplementary Tables (PDF 1503 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Griffiths, K., Ahmed, M., Das, S. et al. Targeting dendritic cells to accelerate T-cell activation overcomes a bottleneck in tuberculosis vaccine efficacy. Nat Commun 7, 13894 (2016). https://doi.org/10.1038/ncomms13894
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms13894
This article is cited by
-
Immune evasion and provocation by Mycobacterium tuberculosis
Nature Reviews Microbiology (2022)
-
A multiplex implantable microdevice assay identifies synergistic combinations of cancer immunotherapies and conventional drugs
Nature Biotechnology (2022)
-
Self-adjuvanting nanovaccines boost lung-resident CD4+ T cell immune responses in BCG-primed mice
npj Vaccines (2022)
-
The possible puzzles of BCG vaccine in protection against COVID-19 infection
The Egyptian Journal of Bronchology (2021)
-
MAIT cell-directed therapy of Mycobacterium tuberculosis infection
Mucosal Immunology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.