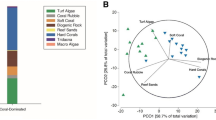
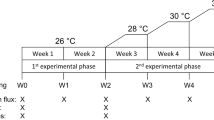
Abstract
Mass coral bleaching, resulting from the breakdown of coral–algal symbiosis has been identified as the most severe threat to coral reef survival on a global scale1. Regionally, nutrient enrichment of reef waters is often associated with a significant loss of coral cover and diversity2. Recently, increased dissolved inorganic nitrogen concentrations have been linked to a reduction of the temperature threshold of coral bleaching3, a phenomenon for which no mechanistic explanation is available. Here we show that increased levels of dissolved inorganic nitrogen in combination with limited phosphate concentrations result in an increased susceptibility of corals to temperature- and light-induced bleaching. Mass spectrometric analyses of the algal lipidome revealed a marked accumulation of sulpholipids under these conditions. Together with increased phosphatase activities, this change indicates that the imbalanced supply of dissolved inorganic nitrogen results in phosphate starvation of the symbiotic algae. Based on these findings we introduce a conceptual model that links unfavourable ratios of dissolved inorganic nutrients in the water column with established mechanisms of coral bleaching. Notably, this model improves the understanding of the detrimental effects of coastal nutrient enrichment on coral reefs, which is urgently required to support knowledge-based management strategies to mitigate the effects of climate change.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hughes, T. P. et al. Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933 (2003).
Fabricius, K. E. Effects of terrestrial runoff on the ecology of corals and coral reefs: Review and synthesis. Mar. Pollut. Bull. 50, 125–146 (2005).
Wooldridge, S. A. Water quality and coral bleaching thresholds: Formalising the linkage for the inshore reefs of the Great Barrier Reef, Australia. Mar. Pollut. Bull. 58, 745–751 (2009).
Dubinsky, Z. & Jokiel, P. L. Ratio of energy and nutrient fluxes regulates symbiosis between zooxanthellae and corals. Pacif. Sci. 48, 313–324 (1994).
Tchernov, D. et al. Apoptosis and the selective survival of host animals following thermal bleaching in zooxanthellate corals. Proc. Natl Acad. Sci. USA 108, 9905–9909 (2011).
Szmant, A. M. Nutrient enrichment on coral reefs: Is it a major cause of coral reef decline? Estuaries 25, 743–766 (2002).
Atkinson, M. J., Carlson, B. & Crow, G. L. Coral growth in high-nutrient, low-pH seawater: A case study of corals cultured at the Waikiki Aquarium, Honolulu, Hawaii. Coral Reefs 14, 215–223 (1995).
Bongiorni, L., Shafir, S., Angel, D. & Rinkevich, B. Survival, growth and gonad development of two hermatypic corals subjected to in situ fish-farm nutrient enrichment. Mar. Ecol. Prog. Ser. 253, 137–144 (2003).
Brodie, J., Devlin, M., Haynes, D. & Waterhouse, J. Assessment of the eutrophication status of the Great Barrier Reef lagoon (Australia). Biogeochemistry 106, 281–302 (2011).
Parkhill, J-P., Maillet, G. & Cullen, J. J. Fluorescence-based maximal quantum yield for PSII as a diagnostic of nutrient stress. J. Phycol. 37, 517–529 (2001).
Miller, D. J. & Yellowlees, D. Inorganic nitrogen uptake by symbiotic marine cnidarians: A critical review. Proc. R. Soc. Lond. B 237, 109–125 (1989).
Muscatine, L., Falkowski, P. G., Dubinsky, Z., Cook, P. A. & McCloskey, L. R. The effect of external nutrient resources on the population dynamics of zooxanthellae in a reef coral. Proc. R. Soc. Lond. B 236, 311–324 (1989).
Rahav, O., Dubinsky, Z., Achituv, Y. & Falkowski, P. G. Ammonium metabolism in the zooxanthellate coral, stylophora pistillata. Proc. R. Soc. Lond. B 236, 325–337 (1989).
Berkelmans, R. & Willis, B. L. Seasonal and local spatial patterns in the upper thermal limits of corals on the inshore Central Great Barrier Reef. Coral Reefs 18, 219–228 (1999).
D’Angelo, C. & Wiedenmann, J. An experimental mesocosm for long-term studies of reef corals. J. Mar. Biol. Assoc. UK 92, 769–775 (2012).
Warner, M. E., Lesser, M. P. & Ralph, P. J. in Chlorophyll Fluorescence in Aquatic Sciences: Methods and Applications (eds Suggett, D. J., Prasil, O. & Borowitzka, M.) (Springer, 2010).
Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshwat. Res. 50, 839–866 (1999).
Jones, R. J. & Hoegh-Guldberg, O. Diurnal changes in the photochemical efficiency of the symbiotic dinoflagellates (Dinophyceae) of corals: Photoprotection, photoinactivation and the relationship to coral bleaching. Plant Cell Environ. 24, 89–99 (2001).
Smith, D. J., Suggett, D. J. & Baker, N. R. Is photoinhibition of zooxanthellae photosynthesis the primary cause of thermal bleaching in corals? Glob. Change Biol. 11, 1–11 (2005).
Annis, E. R. & Cook, C. B. Alkaline phosphatase activity in symbiotic dinoflagellates (zooxanthellae) as a biological indicator of environmental phosphate exposure. Mar. Ecol. Prog. Ser. 245, 11–20 (2002).
Frentzen, M. Phosphatidylglycerol and sulphoquinovosyldiacylglycerol: Anionic membrane lipids and phosphate regulation. Curr. Opin. Plant Biol. 7, 270–276 (2004).
Warner, M. E., Fitt, W. K. & Schmidt, G. W. Damage to photosystem II in symbiotic dinoflagellates: A determinant of coral bleaching. Proc. Natl Acad. Sci. USA 96, 8007–8012 (1999).
Tchernov, D. et al. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc. Natl Acad. Sci. USA 101, 13531–13535 (2004).
Banaszak, A. T., Ayala-Schiaffino, B. N., Rodriguez-Roman, A., Enriquez, J. & Iglesias-Prieto, R. Response of Millepora alcicornis (Milleporina : Milleporidae) to two bleaching events at Puerto Morelos reef, Mexican Caribbean. Rev. Biol. Trop. 51, 57–66 (2003).
Lesser, M. P. Oxidative stress in marine environments: Biochemistry and physiological ecology. Annu. Rev. Physiol. 68, 253–278 (2006).
Shick, J. M., Iglic, K., Wells, C. G., Trick, J. D. & Dunlap, W. C. Responses to iron limitation in two colonies of Stylophora pistillata exposed to high temperature: Implications for coral bleaching. Limnol. Oceanogr. 56, 813–828 (2011).
Hartle-Mougiou, K. et al. Diversity of zooxanthellae from corals and sea anemones after long-term aquarium culture. J. Mar. Biol. Assoc. UK 92, 687–691 (2012).
Gordon, L. I., Jennings, J. C. J., Ross, A. A. & Krest, J. M. A suggested protocol for continuous flow automated analysis of seawater nutrients in the WOCE Hydrographic Program and the Joint Global Ocean Fluxes Study. OSU Coll. of Oc. Descr. Chem. Oc. Grp. Tech. Rpt. 92-1 (1992).
Patey, M. D. et al. Determination of nitrate and phosphate in seawater at nanomolar concentrations. Trends Anal. Chem. 27, 169–182 (2008).
D’Angelo, C. et al. Blue light regulation of host pigment in reef-building corals. Mar. Ecol. Prog. Ser. 364, 97–106 (2008).
Acknowledgements
The study was financially supported by the Deutsche Forschungsgemeinschaft (DFG Wi1990/2-1 to J.W.), NERC (NE/H012303/1, NE/I01683X/1 to J.W., studentship to E.G.S./J.W.), SENSEnet (EU FP7, PITN-GA-2009-237868) to F.E.L., and EPSRC/IFLS (Bridging the Gap grant to J.W., A.D.P. and E.P.A.). We acknowledge the Tropical Marine Centre, London, UK and Tropic Marin, Wartenberg, Germany for sponsoring the coral reef laboratory.
Author information
Authors and Affiliations
Contributions
J.W. and C.D.A. contributed equally, conceiving the conceptual model of nutrient-stress-mediated coral bleaching, the experimental strategies and set-up. J.W., C.D.A., E.G.S., A.N.H. and F-E.L. conducted the experiments. All authors contributed to the data analyses. J.W., C.D.A., E.G.S., A.N.H., A.D.P. and E.P.A. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 617 kb)
Rights and permissions
About this article
Cite this article
Wiedenmann, J., D’Angelo, C., Smith, E. et al. Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nature Clim Change 3, 160–164 (2013). https://doi.org/10.1038/nclimate1661
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nclimate1661
This article is cited by
-
Toward a Multi-stressor Theory for Coral Reefs in a Changing World
Ecosystems (2024)
-
Importance of depth and temperature variability as drivers of coral symbiont composition despite a mass bleaching event
Scientific Reports (2023)
-
Moderate chlorophyll-a environments reduce coral bleaching during thermal stress in Yap, Micronesia
Scientific Reports (2023)
-
Reef-building corals farm and feed on their photosynthetic symbionts
Nature (2023)
-
Climate change implications for the Arafura and Timor Seas region: assessing vulnerability of marine systems to inform management and conservation
Climatic Change (2023)