Abstract
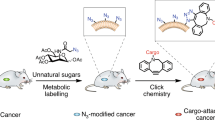
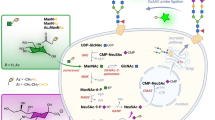
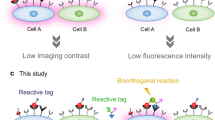
Distinguishing cancer cells from normal cells through surface receptors is vital for cancer diagnosis and targeted therapy. Metabolic glycoengineering of unnatural sugars provides a powerful tool to manually introduce chemical receptors onto the cell surface; however, cancer-selective labeling still remains a great challenge. Herein we report the design of sugars that can selectively label cancer cells both in vitro and in vivo. Specifically, we inhibit the cell-labeling activity of tetraacetyl-N-azidoacetylmannosamine (Ac4ManAz) by converting its anomeric acetyl group to a caged ether bond that can be selectively cleaved by cancer-overexpressed enzymes and thus enables the overexpression of azido groups on the surface of cancer cells. Histone deacetylase and cathepsin L-responsive acetylated azidomannosamine, one such enzymatically activatable Ac4ManAz analog developed, mediated cancer-selective labeling in vivo, which enhanced tumor accumulation of a dibenzocyclooctyne–doxorubicin conjugate via click chemistry and enabled targeted therapy against LS174T colon cancer, MDA-MB-231 triple-negative breast cancer and 4T1 metastatic breast cancer in mice.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hynes, R.O. Integrins: a family of cell surface receptors. Cell 48, 549–554 (1987).
Aruffo, A., Stamenkovic, I., Melnick, M., Underhill, C.B. & Seed, B. CD44 is the principal cell surface receptor for hyaluronate. Cell 61, 1303–1313 (1990).
Wright, S.D., Ramos, R.A., Tobias, P.S., Ulevitch, R.J. & Mathison, J.C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249, 1431–1433 (1990).
Nusser, Z. et al. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron 21, 545–559 (1998).
Cull-Candy, S., Brickley, S. & Farrant, M. NMDA receptor subunits: diversity, development and disease. Curr. Opin. Neurobiol. 11, 327–335 (2001).
Raff, M.C. et al. Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res. 174, 283–308 (1979).
Liaw, D. et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat. Genet. 16, 64–67 (1997).
Mombaerts, P. et al. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell 75, 274–282 (1993).
Puffenberger, E.G. et al. A missense mutation of the endothelin-B receptor gene in multigenic Hirschsprung's disease. Cell 79, 1257–1266 (1994).
Slamon, D.J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001).
Vogel, C.L. et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J. Clin. Oncol. 20, 719–726 (2002).
Romond, E.H. et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 353, 1673–1684 (2005).
Tang, L. et al. Aptamer-functionalized, ultra-small, monodisperse silica nanoconjugates for targeted dual-modal imaging of lymph nodes with metastatic tumors. Angew. Chem. Int. Ed. Engl. 51, 12721–12726 (2012).
Keefe, A.D., Pai, S. & Ellington, A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 9, 537–550 (2010).
Santra, S., Kaittanis, C., Santiesteban, O.J. & Perez, J.M. Cell-specific, activatable, and theranostic prodrug for dual-targeted cancer imaging and therapy. J. Am. Chem. Soc. 133, 16680–16688 (2011).
Slamon, D.J. et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244, 707–712 (1989).
Goldhirsch, A. et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet 382, 1021–1028 (2013).
Hudziak, R.M., Schlessinger, J. & Ullrich, A. Increased expression of the putative growth factor receptor p185HER2 causes transformation and tumorigenesis of NIH 3T3 cells. Proc. Natl. Acad. Sci. USA 84, 7159–7163 (1987).
Hartley, J.L., Temple, G.F. & Brasch, M.A. DNA cloning using in vitro site-specific recombination. Genome Res. 10, 1788–1795 (2000).
Prescher, J.A., Dube, D.H. & Bertozzi, C.R. Chemical remodelling of cell surfaces in living animals. Nature 430, 873–877 (2004).
Prescher, J.A. & Bertozzi, C.R. Chemistry in living systems. Nat. Chem. Biol. 1, 13–21 (2005).
Lee, S. et al. Chemical tumor-targeting of nanoparticles based on metabolic glycoengineering and click chemistry. ACS Nano 8, 2048–2063 (2014).
Koo, H. et al. Bioorthogonal copper-free click chemistry in vivo for tumor-targeted delivery of nanoparticles. Angew. Chem. Int. Ed. Engl. 51, 11836–11840 (2012).
Xie, R., Hong, S., Feng, L., Rong, J. & Chen, X. Cell-selective metabolic glycan labeling based on ligand-targeted liposomes. J. Am. Chem. Soc. 134, 9914–9917 (2012).
Xie, R. et al. Targeted imaging and proteomic analysis of tumor-associated glycans in living animals. Angew. Chem. Int. Ed. Engl. 53, 14082–14086 (2014).
Xie, R. et al. In vivo metabolic labeling of sialoglycans in the mouse brain by using a liposome-assisted bioorthogonal reporter strategy. Proc. Natl. Acad. Sci. USA 113, 5173–5178 (2016).
Saks, M.E. et al. An engineered Tetrahymena tRNAGln for in vivo incorporation of unnatural amino acids into proteins by nonsense suppression. J. Biol. Chem. 271, 23169–23175 (1996).
Liu, W., Brock, A., Chen, S., Chen, S. & Schultz, P.G. Genetic incorporation of unnatural amino acids into proteins in mammalian cells. Nat. Methods 4, 239–244 (2007).
Link, A.J., Mock, M.L. & Tirrell, D.A. Non-canonical amino acids in protein engineering. Curr. Opin. Biotechnol. 14, 603–609 (2003).
Rodriguez, E.A., Lester, H.A. & Dougherty, D.A. In vivo incorporation of multiple unnatural amino acids through nonsense and frameshift suppression. Proc. Natl. Acad. Sci. USA 103, 8650–8655 (2006).
Laughlin, S.T. & Bertozzi, C.R. Metabolic labeling of glycans with azido sugars and subsequent glycan-profiling and visualization via Staudinger ligation. Nat. Protoc. 2, 2930–2944 (2007).
Breidenbach, M.A. et al. Targeted metabolic labeling of yeast N-glycans with unnatural sugars. Proc. Natl. Acad. Sci. USA 107, 3988–3993 (2010).
Luchansky, S.J. et al. Constructing azide-labeled cell surfaces using polysaccharide biosynthetic pathways. Methods Enzymol. 362, 249–272 (2003).
Chang, P.V., Dube, D.H., Sletten, E.M. & Bertozzi, C.R. A strategy for the selective imaging of glycans using caged metabolic precursors. J. Am. Chem. Soc. 132, 9516–9518 (2010).
Hao, J., Vann, W.F., Hinderlich, S. & Sundaramoorthy, M. Elimination of 2-keto-3-deoxy-D-glycero-D-galacto-nonulosonic acid 9-phosphate synthase activity from human N-acetylneuraminic acid 9-phosphate synthase by a single mutation. Biochem. J. 397, 195–201 (2006).
Gunawan, J. et al. Structural and mechanistic analysis of sialic acid synthase NeuB from Neisseria meningitidis in complex with Mn2+, phosphoenolpyruvate, and N-acetylmannosaminitol. J. Biol. Chem. 280, 3555–3563 (2005).
Il'ichev, Y.V., Schwörer, M.A. & Wirz, J. Photochemical reaction mechanisms of 2-nitrobenzyl compounds: methyl ethers and caged ATP. J. Am. Chem. Soc. 126, 4581–4595 (2004).
Chauhan, S.S., Goldstein, L.J. & Gottesman, M.M. Expression of cathepsin L in human tumors. Cancer Res. 51, 1478–1481 (1991).
Witt, O., Deubzer, H.E., Milde, T. & Oehme, I. HDAC family: what are the cancer relevant targets? Cancer Lett. 277, 8–21 (2009).
Ueki, N., Lee, S., Sampson, N.S. & Hayman, M.J. Selective cancer targeting with prodrugs activated by histone deacetylases and a tumour-associated protease. Nat. Commun. 4, 2735 (2013).
Bonfils, C. et al. Evaluation of the pharmacodynamic effects of MGCD0103 from preclinical models to human using a novel HDAC enzyme assay. Clin. Cancer Res. 14, 3441–3449 (2008).
Wegener, D., Wirsching, F., Riester, D. & Schwienhorst, A. A fluorogenic histone deacetylase assay well suited for high-throughput activity screening. Chem. Biol. 10, 61–68 (2003).
Lodish, H. et al. in Molecular Cell Biology 4th edn. Ch. 20.2 (W.H. Freeman, 2000).
Malinoff, H.L. & Wicha, M.S. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J. Cell Biol. 96, 1475–1479 (1983).
Dent, R. et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin. Cancer Res. 13, 4429–4434 (2007).
Foulkes, W.D., Smith, I.E. & Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 363, 1938–1948 (2010).
Steeg, P.S. Tumor metastasis: mechanistic insights and clinical challenges. Nat. Med. 12, 895–904 (2006).
Yan, S., Sameni, M. & Sloane, B.F. Cathepsin B and human tumor progression. Biol. Chem. 379, 113–123 (1998).
Deller, M.C. & Yvonne Jones, E. Cell surface receptors. Curr. Opin. Struct. Biol. 10, 213–219 (2000).
Lehmann, B.D. et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Invest. 121, 2750–2767 (2011).
Dubowchik, G.M. et al. Cathepsin B-labile dipeptide linkers for lysosomal release of doxorubicin from internalizing immunoconjugates: model studies of enzymatic drug release and antigen-specific in vitro anticancer activity. Bioconjug. Chem. 13, 855–869 (2002).
Tang, L. et al. Investigating the optimal size of anticancer nanomedicine. Proc. Natl. Acad. Sci. USA 111, 15344–15349 (2014).
Acknowledgements
J.C. acknowledges support from the United States National Institute of Health (Director's New Innovator Award 1DP2OD007246), which partially supported the in vivo part of the research, and National Science Foundation (DMR 1309525), which partially supported the chemical design and synthesis of the work. L.Y. acknowledges the support from the National Natural Science Foundation of China (51403145 and 51573123), the Ministry of Science and Technology of China (2016YFA0201200), the Collaborative Innovation Center of Suzhou Nano Science and Technology, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). X.C. acknowledges the support from the National Natural Science Foundation of China (51528303). H.W. was supported via a Howard Hughes Medical Institute International Student Research Fellowship. K.C. and Q.Y. acknowledge Beckman Institute Graduate Fellowship support at the University of Illinois at Urbana–Champaign. K.C., Q.Y., and L.T. acknowledge support from the NIH National Cancer Institute Alliance for Nanotechnology in Cancer “Midwest Cancer Nanotechnology Training Center” Grant R25 CA154015A. R.W. acknowledges the support of a CSTAR/T32 Fellowship through the NIH T32 Tissue Microenvironment Training Program. We acknowledge R. Tong and V. Mirshafiee for their early work related to the design of this project. We also acknowledge H. Ying and Y. Zhang for their useful discussions.
Author information
Authors and Affiliations
Contributions
J.C. conceived the original concept of the two-step targeting strategy and supervised the entire project. J.C., H.W., Q.Y. and L.T. initiated this project. H.W. demonstrated the controlled labeling strategy using ether-caged Ac3ManAz derivatives and designed DCL-AAM under the supervision of J.C. H.W. performed the initial synthesis of DCL-AAM. R.W. and K.C. enabled the synthesis of high-purity DCL-AAM. H.W. performed the experiments of flow cytometry and confocal imaging with the help of Y.L. and J.Y. H.W., M.X., Y.S., X.Z. and E.J.C. designed and performed in vivo imaging studies under the supervision of J.C., I.T.D., L.W.D. and S.A.B. H.W., H.H., R.W., Z.W. and K.C. designed and performed tumor efficacy studies under the supervision of J.C., L.Y., X.C., S.L. and T.M.F. H.W., J.C., L.Y., S.L., T.M.F. and X.C. analyzed data. L.Y., X.C., T.M.F. and S.L. provided other expertise and critical feedback. H.W., J.C., L.Y., S.L. and T.M.F. wrote the paper with input from other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results and Supplementary Figures 1–17. (PDF 7431 kb)
Supplementary Note
Synthetic Procedures. (PDF 697 kb)
Rights and permissions
About this article
Cite this article
Wang, H., Wang, R., Cai, K. et al. Selective in vivo metabolic cell-labeling-mediated cancer targeting. Nat Chem Biol 13, 415–424 (2017). https://doi.org/10.1038/nchembio.2297
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2297
This article is cited by
-
An in situ dual-anchoring strategy for enhanced immobilization of PD-L1 to treat autoimmune diseases
Nature Communications (2023)
-
Metabolic glycan labeling immobilizes dendritic cell membrane and enhances antitumor efficacy of dendritic cell vaccine
Nature Communications (2023)
-
Metabolic tagging of extracellular vesicles and development of enhanced extracellular vesicle based cancer vaccines
Nature Communications (2023)
-
Capturing nascent extracellular vesicles by metabolic glycan labeling-assisted microfluidics
Nature Communications (2023)
-
Bioorthogonal chemistry based on-demand drug delivery system in cancer therapy
Frontiers of Chemical Science and Engineering (2023)