Abstract
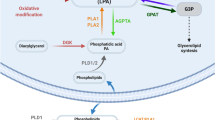
Lysophosphatidylserines (lyso-PSs) are a class of signaling lipids that regulate immunological and neurological processes. The metabolism of lyso-PSs remains poorly understood in vivo. Recently, we determined that ABHD12 is a major brain lyso-PS lipase, implicating lyso-PSs in the neurological disease polyneuropathy, hearing loss, ataxia, retinitis pigmentosa and cataract (PHARC), which is caused by null mutations in the ABHD12 gene. Here, we couple activity-based profiling with pharmacological and genetic methods to annotate the poorly characterized enzyme ABHD16A as a phosphatidylserine (PS) lipase that generates lyso-PS in mammalian systems. We describe a small-molecule inhibitor of ABHD16A that depletes lyso-PSs from cells, including lymphoblasts derived from subjects with PHARC. In mouse macrophages, disruption of ABHD12 and ABHD16A respectively increases and decreases both lyso-PSs and lipopolysaccharide-induced cytokine production. Finally, Abhd16a−/− mice have decreased brain lyso-PSs, which runs counter to the elevation in lyso-PS in Abhd12−/− mice. Our findings illuminate an ABHD16A-ABHD12 axis that dynamically regulates lyso-PS metabolism in vivo, designating these enzymes as potential targets for treating neuroimmunological disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Rivera, R. & Chun, J. Biological effects of lysophospholipids. Rev. Physiol. Biochem. Pharmacol. 160, 25–46 (2008).
Makide, K. et al. Novel lysophospholipid receptors; their structure and function. J. Lipid Res. 55, 1986–1995 (2014).
Rosen, H., Stevens, R.C., Hanson, M., Roberts, E. & Oldstone, M.B. Sphingosine-1-phosphate and its receptors: structure, signaling, and influence. Annu. Rev. Biochem. 82, 637–662 (2013).
Lynch, K.R. & Macdonald, T.L. Sphingosine 1-phosphate chemical biology. Biochim. Biophys. Acta 1781, 508–512 (2008).
van der Kleij, D. et al. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J. Biol. Chem. 277, 48122–48129 (2002).
Inoue, A. et al. TGFα shedding assay: an accurate and versatile method for detecting GPCR activation. Nat. Methods 9, 1021–1029 (2012).
Sugita, K., Yamamura, C., Tabata, K. & Fujita, N. Expression of orphan G-protein coupled receptor GPR174 in CHO cells induced morphological changes and proliferation delay via increasing intracellular cAMP. Biochem. Biophys. Res. Commun. 430, 190–195 (2013).
Chu, X. et al. An X chromosome-wide association analysis identifies variants in GPR174 as a risk factor for Graves' disease. J. Med. Genet. 50, 479–485 (2013).
Szymański, K. et al. rs3827440, a nonsynonymous single nucleotide polymorphism within GPR174 gene in X chromosome, is associated with Graves' disease in Polish Caucasian population. Tissue Antigens 83, 41–44 (2014).
Frasch, S.C. & Bratton, D.L. Emerging roles for lysophosphatidylserine in resolution of inflammation. Prog. Lipid Res. 51, 199–207 (2012).
Park, K.S., Lee, H.Y., Kim, M.K., Shin, E.H. & Bae, Y.S. Lysophosphatidylserine stimulates leukemic cells but not normal leukocytes. Biochem. Biophys. Res. Commun. 333, 353–358 (2005).
Lloret, S. & Moreno, J.J. Ca2+ influx, phosphoinositide hydrolysis, and histamine release induced by lysophosphatidylserine in mast cells. J. Cell. Physiol. 165, 89–95 (1995).
Lee, S.Y. et al. Lysophosphatidylserine stimulates chemotactic migration in U87 human glioma cells. Biochem. Biophys. Res. Commun. 374, 147–151 (2008).
Knowlden, S. & Georas, S.N. The autotaxin-LPA axis emerges as a novel regulator of lymphocyte homing and inflammation. J. Immunol. 192, 851–857 (2014).
Billich, A. & Baumruker, T. Sphingolipid metabolizing enzymes as novel therapeutic targets. Subcell. Biochem. 49, 487–522 (2008).
Blankman, J.L., Long, J.Z., Trauger, S.A., Siuzdak, G. & Cravatt, B.F. ABHD12 controls brain lysophosphatidylserine pathways that are deregulated in a murine model of the neurodegenerative disease PHARC. Proc. Natl. Acad. Sci. USA 110, 1500–1505 (2013).
Fiskerstrand, T. et al. Mutations in ABHD12 cause the neurodegenerative disease PHARC: an inborn error of endocannabinoid metabolism. Am. J. Hum. Genet. 87, 410–417 (2010).
Chen, D.H. et al. Two novel mutations in ABHD12: expansion of the mutation spectrum in PHARC and assessment of their functional effects. Hum. Mutat. 34, 1672–1678 (2013).
Hosono, H. et al. Phosphatidylserine-specific phospholipase A1 stimulates histamine release from rat peritoneal mast cells through production of 2-acyl-1-lysophosphatidylserine. J. Biol. Chem. 276, 29664–29670 (2001).
Aoki, J., Nagai, Y., Hosono, H., Inoue, K. & Arai, H. Structure and function of phosphatidylserine-specific phospholipase A1. Biochim. Biophys. Acta 1582, 26–32 (2002).
Kelleher, J.A. & Sun, G.Y. Enzymic hydrolysis of arachidonoyl-phospholipids by rat brain synaptosomes. Neurochem. Int. 7, 825–831 (1985).
Simon, G.M. & Cravatt, B.F. Activity-based proteomics of enzyme superfamilies: serine hydrolases as a case study. J. Biol. Chem. 285, 11051–11055 (2010).
Borgström, B. Mode of action of tetrahydrolipstatin: a derivative of the naturally occurring lipase inhibitor lipstatin. Biochim. Biophys. Acta 962, 308–316 (1988).
Hoover, H.S., Blankman, J.L., Niessen, S. & Cravatt, B.F. Selectivity of inhibitors of endocannabinoid biosynthesis evaluated by activity-based protein profiling. Bioorg. Med. Chem. Lett. 18, 5838–5841 (2008).
Baggelaar, M.P. et al. Development of an activity-based probe and in silico design reveal highly selective inhibitors for diacylglycerol lipase-α in brain. Angew. Chem. Int. Edn. Engl. 52, 12081–12085 (2013).
Pemble, C.W.T., Johnson, L.C., Kridel, S.J. & Lowther, W.T. Crystal structure of the thioesterase domain of human fatty acid synthase inhibited by Orlistat. Nat. Struct. Mol. Biol. 14, 704–709 (2007).
Dekker, F.J. et al. Small-molecule inhibition of APT1 affects Ras localization and signaling. Nat. Chem. Biol. 6, 449–456 (2010).
Ortar, G. et al. Tetrahydrolipstatin analogues as modulators of endocannabinoid 2-arachidonoylglycerol metabolism. J. Med. Chem. 51, 6970–6979 (2008).
Ngai, M.H. et al. Click-based synthesis and proteomic profiling of lipstatin analogues. Chem. Commun. (Camb.) 46, 8335–8337 (2010).
Camara, K., Kamat, S., Lasota, C.C., Cravatt, B.F. & Howell, A.R. Combining cross-metathesis and activity-based protein profiling: new β-lactone motifs for targeting serine hydrolases. Bioorg. Med. Chem. Lett. 25, 317–321 (2015).
Jessani, N. et al. A streamlined platform for high-content functional proteomics of primary human specimens. Nat. Methods 2, 691–697 (2005).
Adibekian, A. et al. Click-generated triazole ureas as ultrapotent in vivo–active serine hydrolase inhibitors. Nat. Chem. Biol. 7, 469–478 (2011).
Liu, Y., Patricelli, M.P. & Cravatt, B.F. Activity-based protein profiling: the serine hydrolases. Proc. Natl. Acad. Sci. USA 96, 14694–14699 (1999).
Blankman, J.L., Simon, G.S. & Cravatt, B.F. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 14, 1347–1356 (2007).
Hua, X., Sakai, J., Ho, Y.K., Goldstein, J.L. & Brown, M.S. Hairpin orientation of sterol regulatory element-binding protein-2 in cell membranes as determined by protease protection. J. Biol. Chem. 270, 29422–29427 (1995).
Vance, J.E. & Tasseva, G. Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. Biochim. Biophys. Acta 1831, 543–554 (2013).
Rossol, M. et al. LPS-induced cytokine production in human monocytes and macrophages. Crit. Rev. Immunol. 31, 379–446 (2011).
Boersema, P.J., Raijmakers, R., Lemeer, S., Mohammed, S. & Heck, A.J. Multiplex peptide stable isotope dimethyl labeling for quantitative proteomics. Nat. Protoc. 4, 484–494 (2009).
Wilson-Grady, J.T., Haas, W. & Gygi, S.P. Quantitative comparison of the fasted and re-fed mouse liver phosphoproteomes using lower pH reductive dimethylation. Methods 61, 277–286 (2013).
Kim, H.Y., Huang, B.X. & Spector, A.A. Phosphatidylserine in the brain: metabolism and function. Prog. Lipid Res. 56C, 1–18 (2014).
Spies, T., Blanck, G., Bresnahan, M., Sands, J. & Strominger, J.L. A new cluster of genes within the human major histocompatibility complex. Science 243, 214–217 (1989).
Spies, T., Bresnahan, M. & Strominger, J.L. Human major histocompatibility complex contains a minimum of 19 genes between the complement cluster and HLA-B. Proc. Natl. Acad. Sci. USA 86, 8955–8958 (1989).
Frasch, S.C. et al. Neutrophils regulate tissue neutrophilia in inflammation via the oxidant-modified lipid lysophosphatidylserine. J. Biol. Chem. 288, 4583–4593 (2013).
Ishii, I., Fukushima, N., Ye, X. & Chun, J. Lysophospholipid receptors: signaling and biology. Annu. Rev. Biochem. 73, 321–354 (2004).
Swaney, J.S. et al. A novel, orally active LPA1 receptor antagonist inhibits lung fibrosis in the mouse bleomycin model. Br. J. Pharmacol. 160, 1699–1713 (2010).
Inloes, J.M. et al. The hereditary spastic paraplegia-related enzyme DDHD2 is a principal brain triglyceride lipase. Proc. Natl. Acad. Sci. USA 111, 14924–14929 (2014).
Hsu, K.L. et al. DAGLβ inhibition perturbs a lipid network involved in macrophage inflammatory responses. Nat. Chem. Biol. 8, 999–1007 (2012).
Washburn, M.P., Wolters, D. & Yates, J.R. III. Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat. Biotechnol. 19, 242–247 (2001).
Weerapana, E. et al. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 468, 790–795 (2010).
Acknowledgements
We are grateful to K. Masuda, A. Viader and K.-L. Hsu for their helpful discussions, guidance and technical expertise in harvesting tissues; G. Simon and J. Blankman for helpful discussions; M. Niphakis for advice on the SAR studies on the β-lactone scaffolds; O. Ulanovskaya for advice on generating the shRNA knockdown cell lines; B. Correia for the guidance on using CIMAGE; C. Joslyn and T. Takei for technical assistance; and M. Lau for contributions to initial synthetic studies. This work was supported by the US National Institutes of Health (DA033760) and the ninth Irving S. Sigal postdoctoral fellowship from the American Chemical Society (to S.S.K.), a Hewitt Foundation for Medical Research fellowship (to W.H.P.), the Department of Veterans Affairs Research Funds (to T.D.B.) and the US National Science Foundation (CHE-0809753 and CHE-1048717 to A.R.H.).
Author information
Authors and Affiliations
Contributions
S.S.K. and B.F.C. conceived the project and designed the experiments. S.S.K. performed the biochemical and cell biological experiments. K.C. and A.R.H. synthesized and chemically characterized all β-lactone compounds. D.-H.C. and T.D.B. generated the LCL lines from the patient and controls in this study. W.H.P. assisted with chemical and biochemical studies, and M.M.D. performed the ABPP-reductive dimethylation experiments. S.S.K. and B.F.C. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
B.F.C. is a founder and advisor to Abide Therapeutics, a biotechnology company interested in developing serine hydrolase inhibitors and therapeutics.
Supplementary information
Supplementary Text and Figures
Supplementary Results and Supplementary Figures 1–32. (PDF 27746 kb)
Supplementary Table 1
Supplementary Table 1 (XLSX 130 kb)
Supplementary Table 2
Supplementary Table 2 (XLSX 28 kb)
Supplementary Table 3
Supplementary Table 3 (XLSX 6678 kb)
Supplementary Note
Supplementary Note (PDF 1775 kb)
Rights and permissions
About this article
Cite this article
Kamat, S., Camara, K., Parsons, W. et al. Immunomodulatory lysophosphatidylserines are regulated by ABHD16A and ABHD12 interplay. Nat Chem Biol 11, 164–171 (2015). https://doi.org/10.1038/nchembio.1721
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1721
This article is cited by
-
Increase in Cellular Lysophosphatidylserine Content Exacerbates Inflammatory Responses in LPS-Activated Microglia
Neurochemical Research (2022)
-
Mirtronic miR-4646-5p promotes gastric cancer metastasis by regulating ABHD16A and metabolite lysophosphatidylserines
Cell Death & Differentiation (2021)
-
Current Knowledge on the Biology of Lysophosphatidylserine as an Emerging Bioactive Lipid
Cell Biochemistry and Biophysics (2021)
-
The Lysophosphatidylserines—An Emerging Class of Signalling Lysophospholipids
The Journal of Membrane Biology (2020)
-
Lysophosphatidylinositol, an Endogenous Ligand for G Protein-Coupled Receptor 55, Has Anti-inflammatory Effects in Cultured Microglia
Inflammation (2020)