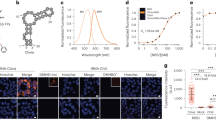
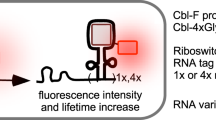
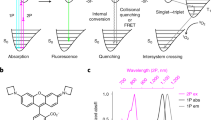
Abstract
Synergistic advances in optical physics, probe design, molecular biology, labeling techniques and computational analysis have propelled fluorescence imaging into new realms of spatiotemporal resolution and sensitivity. This review aims to discuss advances in fluorescent probes and live-cell labeling strategies, two areas that remain pivotal for future advances in imaging technology. Fluorescent protein– and bio-orthogonal–based methods for protein and RNA imaging are discussed as well as emerging bioengineering techniques that enable their expression at specific genomic loci (for example, CRISPR and TALENs). Important attributes that contribute to the success of each technique are emphasized, providing a guideline for future advances in dynamic live-cell imaging.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kanchanawong, P. et al. Nanoscale architecture of integrin-based cell adhesions. Nature 468, 580–584 (2010). An elegant study on the three-dimensional nanostructure of mechanochemical signaling domains using interferometry-based super-resolution imaging.
Toettcher, J.E., Weiner, O.D. & Lim, W.A. Using optogenetics to interrogate the dynamic control of signal transmission by the Ras/Erk module. Cell 155, 1422–1434 (2013). This article demonstrates the power of fluorescence imaging coupled with optogenetics and proteomics for interrogation of frequency-dependent signal transduction.
Hao, N., Budnik, B.A., Gunawardena, J. & O'Shea, E.K. Tunable signal processing through modular control of transcription factor translocation. Science 339, 460–464 (2013).
Machacek, M. et al. Coordination of rho GTPase activities during cell protrusion. Nature 461, 99–103 (2009).
Boch, J. et al. Breaking the code of DNA binding specificity of TAL-type III effectors. Science 326, 1509–1512 (2009).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Lukinavičius, G. et al. A near-infrared fluorophore for live-cell super-resolution microscopy of cellular proteins. Nat. Chem. 5, 132–139 (2013). A bio-orthogonal–compatible silicon-based small-molecule fluorophore provides excellent contrast in the near-infrared and its use in vivo. This paper demonstrates how optimal probe photophysical properties can enable imaging from live-cell super-resolution to thick and scattering tissue samples.
Dean, K.M. et al. Analysis of red-fluorescent proteins provides insight into dark-state conversion and photodegradation. Biophys. J. 101, 961–969 (2011).
Stennett, E.M.S., Ciuba, M.A. & Levitus, M. Photophysical processes in single molecule organic fluorescent probes. Chem. Soc. Rev. 43, 1057–1075 (2014).
Shu, X. et al. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 9, e1001041 (2011).
Shu, X. et al. Mammalian expression of infrared fluorescent proteins engineered from a bacterial phytochrome. Science 324, 804–807 (2009).
Nienhaus, K. & Nienhaus, G.U. Fluorescent proteins for live-cell imaging with super-resolution. Chem. Soc. Rev. 43, 1088 (2014).
Sengupta, P., van Engelenburg, S.B. & Lippincott-Schwartz, J. Superresolution imaging of biological systems using photoactivated localization microscopy. Chem. Rev. 114, 3189–3202 (2014).
Tomosugi, W. et al. An ultramarine fluorescent protein with increased photostability and pH insensitivity. Nat. Methods 6, 351–353 (2009).
Ai, H.W., Shaner, N.C., Cheng, Z., Tsien, R.Y. & Campbell, R.E. Exploration of new chromophore structures leads to the identification of improved blue fluorescent proteins. Biochemistry 46, 5904–5910 (2007).
Goedhart, J. et al. Structure-guided evolution of cyan fluorescent proteins towards a quantum yield of 93%. Nat. Commun. 3, 751 (2012). This article demonstrates how the application of careful selection pressures and sophisticated analysis can continue to improve fluorescent protein performance.
Shaner, N.C. et al. A bright monomeric green fluorescent protein derived from branchiostoma lanceolatum. Nat. Methods 10, 407–409 (2013). A unique computational approach results in the brightest green fluorescent protein yet.
Lam, A.J. et al. Improving FRET dynamic range with bright green and red fluorescent proteins. Nat. Methods 9, 1005–1012 (2012).
Shaner, N.C. et al. Improving the photostability of bright monomeric orange and red fluorescent proteins. Nat. Methods 5, 545–551 (2008).
Subach, O.M., Cranfill, P.J., Davidson, M.W. & Verkhusha, V.V. An enhanced monomeric blue fluorescent protein with the high chemical stability of the chromophore. PLoS ONE 6, e28674 (2011).
Shcherbakova, D.M., Hink, M.A., Joosen, L., Gadella, T.W. & Verkhusha, V.V. An orange fluorescent protein with a large stokes shift for single-excitation multicolor FCCS and FRET imaging. J. Am. Chem. Soc. 134, 7913–7923 (2012).
Davis, L.M., Lubbeck, J.L., Dean, K.M., Palmer, A.E. & Jimenez, R. Microfluidic cell sorter for use in developing red fluorescent proteins with improved photostability. Lab Chip 13, 2320–2327 (2013).
Lubbeck, J.L., Dean, K.M., Ma, H., Palmer, A.E. & Jimenez, R. Microfluidic flow cytometer for quantifying photobleaching of fluorescent proteins in cells. Anal. Chem. 84, 3929–3937 (2012).
Vegh, R. B. et al. Chromophore photoreduction in red fluorescent proteins is responsible for bleaching and phototoxicity. J. Phys. Chem. B. 118, 4527–4534 (2014).
Vogelsang, J. et al. A reducing and oxidizing system minimizes photobleaching and blinking of fluorescent dyes. Angew. Chem. Int. Ed. Engl. 47, 5465–5469 (2008).
Habuchi, S. et al. Evidence for the isomerization and decarboxylation in the photocon-version of the red fluorescent protein DsRed. J. Am. Chem. Soc. 127, 8977–8984 (2005).
Adam, V. et al. Structural basis of X-ray–induced transient photobleaching in a photoactivatable green fluorescent protein. J. Am. Chem. Soc. 131, 18063–18065 (2009).
Konold, P., Regmi, C.K., Chapagain, P.P., Gerstman, B.S. & Jimenez, R. Hydrogen bond flexibility correlates with stokes shift in mPlum variants. J. Phys. Chem. B 118, 2940–2948 (2014).
Drobizhev, M., Tillo, S., Makarov, N.S., Hughes, T.E. & Rebane, A. Color hues in red fluorescent proteins are due to internal quadratic stark effect. J. Phys. Chem. B 113, 12860–12864 (2009).
Costantini, L.M. & Snapp, E.L. Fluorescent proteins in cellular organelles: serious pitfalls and some solutions. DNA Cell Biol. 32, 622–627 (2013).
Shemiakina, I.I. et al. A monomeric red fluorescent protein with low cytotoxicity. Nat. Commun. 3, 1204 (2012).
Subach, F.V. et al. Photoactivation mechanism of PAmCherry based on crystal structures of the protein in the dark and fluorescent states. Proc. Natl. Acad. Sci. USA 106, 21097–21102 (2009).
Filonov, G.S. et al. Bright and stable near-infrared fluorescent protein for in vivo imaging. Nat. Biotechnol. 29, 757–761 (2011).
Piatkevich, K.D., Subach, F.V. & Verkhusha, V.V. Far-red light photoactivatable near-infrared fluorescent proteins engineered from a bacterial phytochrome. Nat. Commun. 4, 2153 (2013).
Filonov, G.S. & Verkhusha, V.V. A near-infrared BiFC reporter for in vivo imaging of protein-protein interactions. Chem. Biol. 20, 1078–1086 (2013).
Kumagai, A. et al. A bilirubin-inducible fluorescent protein from eel muscle. Cell 153, 1602–1611 (2013).
Los, G.V. et al. HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem. Biol. 3, 373–382 (2008).
Gautier, A. et al. An engineered protein tag for multiprotein labeling in living cells. Chem. Biol. 15, 128–136 (2008).
Gallagher, S.S., Sable, J.E., Sheetz, M.P. & Cornish, V.W. An in vivo covalent TMP-tag based on proximity-induced reactivity. ACS Chem. Biol. 4, 547–556 (2009).
Tomat, E., Nolan, E.M., Jaworski, J. & Lippard, S.J. Organelle-specific zinc detection using zinpyr-labeled fusion proteins in live cells. J. Am. Chem. Soc. 130, 15776–15777 (2008).
Srikun, D., Albers, A.E., Nam, C.I., Iavarone, A.T. & Chang, C.J. Organelle-targetable fluorescent probes for imaging hydrogen peroxide in living cells via SNAP-tag protein labeling. J. Am. Chem. Soc. 132, 4455–4465 (2010).
Bannwarth, M. et al. Indo-1 derivatives for local calcium sensing. ACS Chem. Biol. 4, 179–190 (2009).
Prifti, E. et al. A fluorogenic probe for SNAP-tagged plasma membrane proteins based on the solvatochromic molecule Nile red. ACS Chem. Biol. 9, 606–612 (2014).
Bojkowska, K. et al. Measuring in vivo protein half-life. Chem. Biol. 18, 805–815 (2011).
Uttamapinant, C. et al. A fluorophore ligase for site-specific protein labeling inside living cells. Proc. Natl. Acad. Sci. USA. 107, 10914–10919 (2010). This article, using an engineered lipoic acid ligase from E. coli that directly couples 7-hydroxycoumarin to a 13-amino-acid peptide, demonstrates intracellular and minimally perturbative labeling of actin, microtubule associated proteins and synaptic membrane proteins in living cells.
Liu, D.S., Phipps, W.S., Loh, K.H., Howarth, M. & Ting, A.Y. Quantum dot targeting with lipoic acid ligase and HaloTag for single-molecule imaging on living cells. ACS Nano 6, 11080–11087 (2012).
Howarth, M., Takao, K., Hayashi, Y. & Ting, A.Y. Targeting quantum dots to surface proteins in living cells with biotin ligase. Proc. Natl. Acad. Sci. USA 102, 7583–7588 (2005).
Martin, B.R., Giepmans, B.N.G., Adams, S.R. & Tsien, R.Y. Mammalian cell based optimization of the biarsenical-binding tetracysteine motif for improved fluorescence and affinity. Nat. Biotechnol. 23, 1308–1314 (2005).
Cohen, M.S., Zhang, C., Shokat, K.M. & Taunton, J. Structural bioinformatics–based design of selective, irreversible kinase inhibitors. Science 308, 1318–1321 (2005).
Tsukiji, S., Miyagawa, M., Takaoka, Y., Tamura, T. & Hamachi, I. Ligand-directed tosyl chemistry for protein labeling in vivo. Nat. Chem. Biol. 5, 341–343 (2009).
Tamura, T., Kioi, Y., Miki, T., Tsukiji, S. & Hamachi, I. Fluorophore labeling of native FKBP12 by ligand-directed tosyl chemistry allows detection of its molecular interactions in vitro and in living cells. J. Am. Chem. Soc. 135, 6782–6785 (2013).
Song, W., Strack, R.L. & Jaffrey, S.R. Imaging bacterial protein expression using genetically encoded RNA sensors. Nat. Methods 10, 873–875 (2013).
Paige, J.S., Wu, K.Y. & Jaffrey, S.R. RNA mimics of green fluorescent protein. Science 333, 642–646 (2011).
Irannejad, R. et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature 495, 534–538 (2013).
Kirchhofer, A. et al. Modulation of protein properties in living cells using nanobodies. Nat. Struct. Mol. Biol. 17, 133–138 (2010).
Fatica, A. & Bozzoni, I. Long non-coding RNAs: new players in cell differentiation and development. Nat. Rev. Genet. 15, 7–21 (2014).
Brengues, M., Teixeira, D. & Parker, R. Movement of eukaryotic mRNAs between polysomes and cytoplasmic processing bodies. Science 310, 486–489 (2005).
Ramaswami, M., Taylor, J.P. & Parker, R. Altered ribostasis: RNA-protein granules in degenerative disorders. Cell 154, 727–736 (2013).
Rudkin, G.T. & Stollar, B.D. High resolution detection of DNA-RNA hybrids in situ by direct immunofluorescence. Nature 265, 472–473 (1977).
Battich, N., Stoeger, T. & Pelkmans, L. Image-based transcriptomics in thousands of single human cells at single-molecule resolution. Nat. Methods 10, 1127–1133 (2013). This article, using sophisticated reagent delivery, FISH probes based on branched DNA technology and automated image analysis, demonstrates the power of single-molecule genome-wide RNA imaging.
Santangelo, P.J. et al. Single molecule–sensitive probes for imaging RNA in live cells. Nat. Methods 6, 347–349 (2009).
Tyagi, S. & Kramer, F.R. Molecular beacons: probes that fluoresce upon hybridization. Nat. Biotechnol. 14, 303–308 (1996).
Bertrand, E. et al. Localization of ash mRNA particles in living yeast. Mol. Cell 2, 437–445 (1998).
Daigle, N. & Ellenberg, J. λN-GFP: an RNA reporter system for live-cell imaging. Nat. Methods 4, 633–636 (2007).
Alcid, E.A. & Jurica, M.S. A protein-based EM label for RNA identifies the location of exons in spliceosomes. Nat. Struct. Mol. Biol. 15, 213–215 (2008).
Ozawa, T., Natori, Y., Sato, M. & Umezawa, Y. Imaging dynamics of endogenous mitochondrial RNA in single living cells. Nat. Methods 4, 413–419 (2007).
Wilson, C. & Szostak, J.W. Isolation of a fluorophore-specific DNA aptamer with weak redox activity. Chem. Biol. 5, 609–617 (1998).
Strack, R.L., Disney, M.D. & Jaffrey, S.R. A superfolding Spinach2 reveals the dynamic nature of trinucleotide repeat containing RNA. Nat. Methods 10, 1219–1224 (2013). An RNA aptamer with improved folding and thermodynamic stability, Spinach2, enables dynamic live-cell imaging of 'toxic' RNAs.
Lee, J. et al. Combining SELEX screening and rational design to develop light-up fluorophore-RNA aptamer pairs for RNA tagging. ACS Chem. Biol. 5, 1065–1074 (2010).
Sunbul, M. & Jäschke, A. Contact-mediated quenching for RNA imaging in bacteria with a fluorophore-binding aptamer. Angew. Chem. Int. Ed. Engl. 52, 13401–13404 (2013).
Lux, J., Peña, E.J., Bolze, F., Heinlein, M. & Nicoud, J.-F. Malachite green derivatives for two-photon RNA detection. ChemBioChem 13, 1206–1213 (2012).
Han, K.Y., Leslie, B.J., Fei, J., Zhang, J. & Ha, T. Understanding the photophysics of the Spinach-DFHBI RNA aptamer–fluorogen complex to improve live cell RNA imaging. J. Am. Chem. Soc. 135, 19033–19038 (2013).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Qi, L.S. et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183 (2013).
Mali, P., Esvelt, K.M. & Church, G.M. Cas9 as a versatile tool for engineering biology. Nat. Methods 10, 957–963 (2013). A thought-provoking perspective on Cas9-mediated bioengineering.
Chen, B. et al. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell 155, 1479–1491 (2013).
Bogdanove, A.J. & Voytas, D.F. TAL effectors: customizable proteins for DNA targeting. Science 333, 1843–1846 (2011).
Santiago, Y. et al. Targeted gene knockout in mammalian cells by using engineered zinc-finger nucleases. Proc. Natl. Acad. Sci. USA 105, 5809–5814 (2008).
Pattanayak, V., Ramirez, C.L., Joung, J.K. & Liu, D.R. Revealing off-target cleavage specificities of zinc-finger nucleases by in vitro selection. Nat. Methods 8, 765–770 (2011).
Betzig, E. et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, 1642–1645 (2006).
Tang, J., van Panhuys, N., Kastenmüller, W. & Germain, R. The future of immunoimaging-deeper, bigger, more precise, and definitively more colorful. Eur. J. Immunol. 43, 1407–1412 (2013).
Livet, J. et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450, 56–62 (2007).
Micheva, K.D., Busse, B., Weiler, N.C., O'Rourke, N. & Smith, S.J. Single-synapse analysis of a diverse synapse population: proteomic imaging methods and markers. Neuron 68, 639–653 (2010).
Zrazhevskiy, P. & Gao, X. Quantum dot imaging platform for single-cell molecular profiling. Nat Commun. 4, 1619 (2013).
Schubert, W. et al. Analyzing proteome topology and function by automated multidimensional fluorescence microscopy. Nat. Biotechnol. 24, 1270–1278 (2006).
Wang, G. & Smith, S.J. Sub-diffraction limit localization of proteins in volumetric space using Bayesian restoration of fluorescence images from ultrathin specimens. PLOS Comput. Biol. 8, e1002671 (2012).
Angelo, M. et al. Multiplexed ion beam imaging of human breast tumors. Nat. Med. 20, 436–442 (2014).
Gorris, H.H. & Wolfbeis, O.S. Photon-upconverting nanoparticles for optical encoding and multiplexing of cells, biomolecules, and microspheres. Angew. Chem. Int. Ed. Engl. 52, 3584–3600 (2013).
Saar, B.G. et al. Video-rate molecular imaging in vivo with stimulated Raman scattering. Science 330, 1368–1370 (2010).
Gao, L. et al. Noninvasive imaging beyond the diffraction limit of 3D dynamics in thickly fluorescent specimens. Cell 151, 1370–1385 (2012). New selective-plane microscope with improved resolution interrogates single cells and developing embryos with unprecedented sensitivity.
Ahrens, M.B., Orger, M.B., Robson, D.N., Li, J.M. & Keller, P.J. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat. Methods 10, 413–420 (2013).
Puchner, E.M., Walter, J.M., Kasper, R., Huang, B. & Lim, W.A. Counting molecules in single organelles with superresolution microscopy allows tracking of the endosome maturation trajectory. Proc. Natl. Acad. Sci. USA 110, 16015–16020 (2013).
Lee, J.H. et al. Highly multiplexed subcellular RNA sequencing in situ. Science 343, 1360–1363 (2014).
Quail, D.F. & Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 (2013).
Xu, T., Agrawal, A., Abashin, M., Chau, K.J. & Lezec, H.J. All-angle negative refraction and active flat lensing of ultraviolet light. Nature 497, 470–474 (2013).
Mamin, H.J. et al. Nanoscale nuclear magnetic resonance with a nitrogen-vacancy spin sensor. Science 339, 557–560 (2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Dean, K., Palmer, A. Advances in fluorescence labeling strategies for dynamic cellular imaging. Nat Chem Biol 10, 512–523 (2014). https://doi.org/10.1038/nchembio.1556
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1556
This article is cited by
-
Bond-selective fluorescence imaging with single-molecule sensitivity
Nature Photonics (2023)
-
Design of a palette of SNAP-tag mimics of fluorescent proteins and their use as cell reporters
Cell Discovery (2023)
-
Cellpose 2.0: how to train your own model
Nature Methods (2022)
-
Total RNA Synthesis and its Covalent Labeling Innovation
Topics in Current Chemistry (2022)
-
9-Cyanopyronin probe palette for super-multiplexed vibrational imaging
Nature Communications (2021)