Abstract
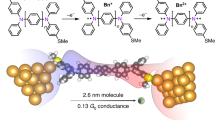
To develop advanced materials for electronic devices, it is of utmost importance to design organic building blocks with tunable functionality and to study their properties at the molecular level. For organic electronic and photovoltaic applications, the ability to vary the nature of charge carriers and so create either electron donors or acceptors is critical. Here we demonstrate that charge carriers in single-molecule junctions can be tuned within a family of molecules that contain electron-deficient thiophene-1,1-dioxide (TDO) building blocks. Oligomers of TDO were designed to increase electron affinity and maintain delocalized frontier orbitals while significantly decreasing the transport gap. Through thermopower measurements we show that the dominant charge carriers change from holes to electrons as the number of TDO units is increased. This results in a unique system in which the charge carrier depends on the backbone length, and provides a new means to tune p- and n-type transport in organic materials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Heeger, A. J. Semiconducting and metallic polymers: the fourth generation of polymeric materials (Nobel lecture). Angew. Chem. Int. Ed. 40, 2591–2611 (2001).
Henson, Z. B., Mullen, K. & Bazan, G. C. Design strategies for organic semiconductors beyond the molecular formula. Nature Chem. 4, 699–704 (2012).
Reddy, P., Jang, S. Y., Segalman, R. A. & Majumdar, A. Thermoelectricity in molecular junctions. Science 315, 1568–1571 (2007).
Bubnova, O. et al. Semi-metallic polymers. Nature Mater. 13, 190–194 (2014).
Schwartz, G. et al. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nature Commun. 4, 1859 (2013).
You, J. et al. A polymer tandem solar cell with 10.6% power conversion efficiency. Nature Commun. 4, 1446 (2013).
Gustafsson, G. et al. Flexible light-emitting diodes made from soluble conducting polymers. Nature 357, 477–479 (1992).
Meijer, E. J. et al. Solution-processed ambipolar organic field-effect transistors and inverters. Nature Mater. 2, 678–682 (2003).
Facchetti, A., Mushrush, M., Katz, H. E. & Marks, T. J. n-type building blocks for organic electronics: a homologous family of fluorocarbon-substituted thiophene oligomers with high carrier mobility. Adv. Mater. 15, 33–38 (2003).
Katz, H. E. et al. A soluble and air-stable organic semiconductor with high electron mobility. Nature 404, 478–481 (2000).
Anthony, J. E., Facchetti, A., Heeney, M., Marder, S. R. & Zhan, X. W. n-type organic semiconductors in organic electronics. Adv. Mater. 22, 3876–3892 (2010).
Chen, W. et al. Aromaticity decreases single-molecule junction conductance. J. Am. Chem. Soc. 136, 918–920 (2014).
Barbarella, G., Pudova, O., Arbizzani, C., Mastragostino, M. & Bongini, A. Oligothiophene-S,S-dioxides: a new class of thiophene-based materials. J. Org. Chem. 63, 1742–1745 (1998).
Camaioni, N., Ridolfi, G., Fattori, V., Favaretto, L. & Barbarella, G. Oligothiophene-S,S-dioxides as a class of electron-acceptor materials for organic photovoltaics. Appl. Phys. Lett. 84, 1901–1903 (2004).
Wei, S. et al. Bandgap engineering through controlled oxidation of polythiophenes. Angew. Chem. Int. Ed. 53, 1832–1836 (2014).
Potash, S. & Rozen, S. New conjugated oligothiophenes containing the unique arrangement of internal adjacent [all]-S,S-oxygenated thiophene fragments. Chem. Eur. J. 19, 5289–5296 (2013).
Dell, E. J. & Campos, L. M. The preparation of thiophene-S,S-dioxides and their role in organic electronics. J. Mater. Chem. 22, 12945–12952 (2012).
Xu, B. Q. & Tao, N. J. J. Measurement of single-molecule resistance by repeated formation of molecular junctions. Science 301, 1221–1223 (2003).
Venkataraman, L. et al. Single-molecule circuits with well-defined molecular conductance. Nano Lett. 6, 458–462 (2006).
Widawsky, J. R., Darancet, P., Neaton, J. B. & Venkataraman, L. Simultaneous determination of conductance and thermopower of single molecule junctions. Nano Lett. 12, 354–358 (2012).
Malen, J. A. et al. Identifying the length dependence of orbital alignment and contact coupling in molecular heterojunctions. Nano Lett. 9, 1164–1169 (2009).
Paulsson, M. & Datta, S. Thermoelectric effect in molecular electronics. Phys. Rev. B 67, 241403 (2003).
Yu, G., Gao, J., Hummelen, J. C., Wudl, F. & Heeger, A. J. Polymer photovoltaic cells—enhanced efficiencies via a network of internal donor–acceptor heterojunctions. Science 270, 1789–1791 (1995).
Park, Y. S. et al. Contact chemistry and single-molecule conductance: a comparison of phosphines, methyl sulfides, and amines. J. Am. Chem. Soc. 129, 15768–15769 (2007).
Amir, E. et al. Synthesis and characterization of soluble low-bandgap oligothiophene-[all]-S,S-dioxides-based conjugated oligomers and polymers. J. Polym. Chem. A 49, 1933–1941 (2011).
Sonar, P., Williams, E. L., Singh, S. P. & Dodabalapur, A. Thiophene–benzothiadiazole–thiophene (D-A-D) based polymers: effect of donor/acceptor moieties adjacent to D-A-D segment on photophysical and photovoltaic properties. J. Mater. Chem. 21, 10532–10541 (2011).
Bolivar-Marinez, L. E., dos Santos, M. C. & Galvao, D. S. Electronic structure of push–pull molecules based on thiophene oligomers. J. Phys. Chem. 100, 11029–11032 (1996).
Capozzi, B. et al. Length-dependent conductance of oligothiophenes. J. Am. Chem. Soc. 136, 10486–10492 (2014).
Yee, S. K., Malen, J. A., Majumdar, A. & Segalman, R. A. Thermoelectricity in fullerene-metal heterojunctions. Nano Lett. 11, 4089–4094 (2011).
Sirringhaus, H. et al. Two-dimensional charge transport in self-organized, high-mobility conjugated polymers. Nature 401, 685–688 (1999).
Bredas, J-L. Mind the gap! Mater. Horiz. 1, 17–19 (2014).
Gonzalez, M. T. et al. Electrical conductance of molecular junctions by a robust statistical analysis. Nano Lett. 6, 2238–2242 (2006).
Dell, E. J. et al. Impact of molecular symmetry on single-molecule conductance. J. Am. Chem. Soc. 135, 11724–11727 (2013).
Kamenetska, M. et al. Formation and evolution of single-molecule junctions. Phys. Rev. Lett. 102, 126803 (2009).
Visoly-Fisher, I. et al. Conductance of a biomolecular wire. Proc. Natl Acad. Sci. USA 103, 8686–8690 (2006).
Meisner, J. S. et al. A single-molecule potentiometer. Nano Lett. 11, 1575–1579 (2011).
Widawsky, J. R. et al. Length-dependent thermopower of highly conducting Au–C bonded single molecule junctions. Nano Lett. 13, 2889–2894 (2013).
Evangeli, C. et al. Engineering the thermopower of C60 molecular junctions. Nano Lett. 13, 2141–2145 (2013).
Fatemi, V., Kamenetska, M., Neaton, J. B. & Venkataraman, L. Environmental control of single-molecule junction transport. Nano Lett. 11, 1988–1992 (2011).
Rozen, S. HOF·CH3CN: probably the best oxygen transfer agent organic chemistry has to offer. Acc. Chem. Res. 47, 2378–2389 (2014).
Stille, J. K. The palladium-catalyzed cross-coupling reactions of organotin reagents with organic electrophiles. Angew. Chem. Int. Ed. Engl. 25, 508–523 (1986).
Bredas, J. L., Silbey, R., Boudreaux, D. S. & Chance, R. R. Chain-length dependence of electronic and electrochemical properties of conjugated systems: polyacetylene, polyphenylene, polythiophene, and polypyrrole. J. Am. Chem. Soc. 105, 6555–6559 (1983).
Acknowledgements
This work was supported primarily by the National Science Foundation under award DMR-1206202. E.J.D. thanks the Howard Hughes Medical Institute, American Australian Association and Dow Chemical Company for International Research Fellowships.
Author information
Authors and Affiliations
Contributions
B.C., E.J.D., L.V. and L.M.C. conceived and designed the experiments. B.C. performed the conductance and thermopower measurements. E.J.D. and J.X. synthesized and characterized the molecules. All authors discussed the results. E.J.D. and B.C. contributed equally to this work. E.J.D., B.C., L.V. and L.M.C. wrote the paper with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1884 kb)
Rights and permissions
About this article
Cite this article
Dell, E., Capozzi, B., Xia, J. et al. Molecular length dictates the nature of charge carriers in single-molecule junctions of oxidized oligothiophenes. Nature Chem 7, 209–214 (2015). https://doi.org/10.1038/nchem.2160
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2160
This article is cited by
-
Tetrathiafulvalenes as anchors for building highly conductive and mechanically tunable molecular junctions
Nature Communications (2022)
-
Investigation of intrinsic charge transport via alkyl thiol molecular electronic junctions with conductive probe atomic force microscopy
Journal of Materials Science: Materials in Electronics (2022)
-
Accurate atomic electron affinities calculated by using anionic Gaussian basis sets
Theoretical Chemistry Accounts (2020)
-
Atomically defined angstrom-scale all-carbon junctions
Nature Communications (2019)
-
Concepts in the design and engineering of single-molecule electronic devices
Nature Reviews Physics (2019)