Abstract
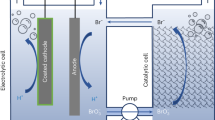
Hydrogen is essential to several key industrial processes and could play a major role as an energy carrier in a future ‘hydrogen economy’. Although the majority of the world's hydrogen supply currently comes from the reformation of fossil fuels, its generation from water using renewables-generated power could provide a hydrogen source without increasing atmospheric CO2 levels. Conventional water electrolysis produces H2 and O2 simultaneously, such that these gases must be generated in separate spaces to prevent their mixing. Herein, using the polyoxometalate H3PMo12O40, we introduce the concept of the electron-coupled-proton buffer (ECPB), whereby O2 and H2 can be produced at separate times during water electrolysis. This could have advantages in preventing gas mixing in the headspaces of high-pressure electrolysis cells, with implications for safety and electrolyser degradation. Furthermore, we demonstrate that temporally separated O2 and H2 production allows greater flexibility regarding the membranes and electrodes that can be used in water-splitting cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
US Department of Energy Hydrogen Analysis Resource Center, Hydrogen Production, Worldwide and US Total Hydrogen Production, http://hydrogen.pnl.gov/cocoon/morf/hydrogen/article/706 (2012).
Schrock, R. R. Reduction of dinitrogen. Proc. Natl Acad. Sci. USA 103, 17087 (2006).
Armaroli, N. & Balzani, V. The hydrogen issue. ChemSusChem 4, 21–36 (2011).
Olah, G. A., Prakash, G. K. S. & Goeppert, A. Anthropogenic chemical carbon cycle for a sustainable future. J. Am. Chem. Soc. 133, 12881–12898 (2011).
Lewis, N. S. & Nocera, D. G. Powering the planet: chemical challenges in solar energy utilization. Proc. Natl Acad. Sci. USA 103, 15729–15735 (2006).
Gust, D., Moore, T. A. & Moore, A. L. Solar fuels via artificial photosynthesis. Acc. Chem. Res. 42, 1890–1898 (2009).
Cook, T. R. et al. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 110, 6474–6502 (2010).
Davis, S. J., Caldeira, K. & Matthews, H. D. Future CO2 emissions and climate change from existing energy infrastructure. Science 329, 1330–1333 (2010).
Häussinger, P., Lohmüller, R. & Watson, A. M. Ullmann's Encyclopedia of Industrial Chemistry, Hydrogen, 2. Production (Wiley-VCH, 2005).
Holladay, J. D., Hu, J., King, D. L. & Wang, Y. An overview of hydrogen production technologies. Catal. Today 139, 244–260 (2009).
Walter, M. G. et al. Solar water splitting cells. Chem. Rev. 110, 6446–6473 (2010).
Chen, X., Shen, S., Guo, L. & Mao, S. S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 110, 6503–6570 (2010).
Kanan, M. W. & Nocera, D. G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 321, 1072–1075 (2008).
Atlam, O. An experimental and modelling study of a photovoltaic/proton-exchange membrane electrolyser system. Int. J. Hydrogen Energy 34, 6589–6595 (2009).
Paul, B. & Andrews, J. Optimal coupling of PV arrays to PEM electrolysers in solar–hydrogen systems for remote area power supply. Int. J. Hydrogen Energy 33, 490–498 (2008).
Barber, J. Photosynthetic energy conversion: natural and artificial. Chem. Soc. Rev. 38, 185–196 (2009).
Funk, J. E. Thermochemical hydrogen production: past and present. Int. J. Hydrogen Energy 26, 185–190 (2001).
Onuki, K., Kubo, S., Terada, A., Sakaba, N. & Hino, R. Thermochemical water-splitting cycle using iodine and sulfur. Energy Environ. Sci. 2, 491–497 (2009).
Tsigdinos, G. A. Heteropoly compounds of molybdenum and tungsten. Top. Curr. Chem. 76, 1–64 (1978).
Maeda, K., Himeno, S., Osakai, T., Saito, A. & Hori, T. A voltammetric study of Keggin-type heteropolymolybdate anions. J. Electroanal. Chem. 364, 149–154 (1994).
Tanaka, N., Unoura, K. & Itabashi, E. Voltammetric and spectroelectrochemical studies of dodecamolybdophosphoric acid in aqueous and water–dioxane solutions at a gold-minigrid optically transparent thin-layer electrode. Inorg. Chem. 21, 1662–1666 (1982).
Hamann, C. H., Hamnett, A. & Vielstich, W. Electrochemistry 2nd edn (Wiley-VCH, 2007).
Merki, D., Fierro, S., Vrubel, H. & Hu, X. Amorphous molybdenum sulfide films as catalysts for electrochemical hydrogen production in water. Chem. Sci. 2, 1262–1267 (2011).
Le Goff, A. et al. From hydrogenases to noble metal–free catalytic nanomaterials for H2 production and uptake. Science 326, 1384–1387 (2009).
McKone, J. R. et al. Evaluation of Pt, Ni, and Ni–Mo electrocatalysts for hydrogen evolution on crystalline Si electrodes. Energy Environ. Sci. 4, 3573–3583 (2011).
Chen, W-F. et al. Hydrogen-evolution catalysts based on non-noble metal nickel–molybdenum nitride nanosheets. Angew. Chem. Int. Ed. 51, 6131–6135 (2012).
Merki, D., Vrubel, H., Rovelli, L., Fierro, S., & Hu, X. Fe, Co, and Ni ions promote the catalytic activity of amorphous molybdenum sulfide films for hydrogen evolution. Chem. Sci. 3, 2515–2525 (2012).
Cobo, S. et al. A Janus cobalt-based catalytic material for electro-splitting of water. Nature Mater. 11, 802–807 (2012).
Miras, H. N. et al. Exploring the structure and properties of transition metal templated {VM17(VO4)2} Dawson-like capsules. Inorg. Chem. 50, 8384–8391 (2011).
Bard, A. J. Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors. J. Photochem. 10, 59–75 (1979).
Darwent, J. R. & Mills, A. Photo-oxidation of water sensitized by WO3 powder. J. Chem. Soc. Faraday Trans. 2 78, 359–367 (1982).
Abe, R., Sayama, K. & Sugihara, H. Development of new photocatalytic water splitting into H2 and O2 using two different semiconductor photocatalysts and a shuttle redox mediator IO3−/I−. J. Phys. Chem. B 109, 16052–16061 (2005).
Maeda, K., Higashi, M., Lu, D., Abe, R. & Domen, K. Efficient nonsacrificial water splitting through two-step photoexcitation by visible light using a modified oxynitride as a hydrogen evolution photocatalyst. J. Am. Chem. Soc. 132, 5858–5868 (2010).
Skolnik, E. G. Compilation of site visit-based technical evaluations of hydrogen projects 1996–2001, Washington DC, http://www.osti.gov/bridge/servlets/purl/815055-bLCRmy/native/815055.pdf (2012).
Pozio, A., Silva, R. F., De Francesco, M. & Giorgi, L. Nafion degradation in PEFCs from end plate iron contamination. Electrochim. Acta 48, 1543–1549 (2003).
Miras, H. N., Wilson, E. F. & Cronin, L. Unravelling the complexities of inorganic and supramolecular self-assembly in solution with electrospray and cryospray mass spectrometry. Chem. Commun. 1297–1311 (2009).
Hernández-Pagán, E. A. et al. Resistance and polarization losses in aqueous buffer-membrane electrolytes for water splitting photoelectrochemical cells. Energy Environ. Sci. 5, 7582–7589 (2012).
Lodi, G., Sivieri, E., De Battisti, A. & Trasatti, S. Ruthenium dioxide-based film electrodes. III. Effect of chemical composition and surface morphology on oxygen evolution in acid solutions. J. Appl. Electrochem. 8, 135–143 (1978).
Burke, L. D., Murphy, O. J., O'Neill, J. F. & Venkatesan, S. The oxygen electrode. Part 8. Oxygen evolution at ruthenium dioxide anodes. J. Chem. Soc. Faraday Trans. 1 73, 1659–1671 (1977).
Sleutels, T. H. J. A., Hamelers, H. V. M., Rozendal, R. A. & Buisman, C. J. N. Ion transport resistance in microbial electrolysis cells with anion and cation exchange membranes. Int. J. Hydrogen Energy 34, 3612–3620 (2009).
Himeno, S. & Takamoto, M. Difference in voltammetric properties between the Keggin-type [XW12O40]n− and [XMo12O40]n− complexes. J. Electroanal. Chem. 528, 170–174 (2002).
Barbir, F. PEM electrolysis for production of hydrogen from renewable energy sources. Solar Energy 78, 661–669 (2005).
Weinstock, I. A. et al. A new environmentally benign technology for transforming wood pulp into paper. Engineering polyoxometalates as catalysts for multiple processes. J. Mol. Catal. A 116, 59–84 (1997).
Sonnen, D. M., Reiner, R. S., Atalla, R. H. & Weinstock, I. A. Degradation of pulp-mill effluent by oxygen and Na5[PV2Mo10O40], a multipurpose delignification and wet air oxidation catalyst. Ind. Eng. Chem. Res. 36, 4134–4142 (1997).
Engstrom, R. C. & Strasser, V. A. Characterization of electrochemically pretreated glassy carbon electrodes. Anal. Chem. 56, 136–141 (1984).
Acknowledgements
This work was supported by the Engineering and Physical Sciences Research Council (UK) and Glasgow Solar Fuels. L.C. thanks the Royal Society/Wolfson Foundation for a Merit Award. M.D.S. thanks the University of Glasgow for a Lord Kelvin Adam Smith Research Fellowship. We are grateful to J. McIver (University of Glasgow) for assistance with the GC headspace analyses, H. N. Miras (University of Glasgow) for mass spectrometry and J. Liddell (University of Glasgow) for manufacturing numerous bespoke electrolysis cells.
Author information
Authors and Affiliations
Contributions
M.D.S. and L.C. conceived the idea, planned experiments and co-wrote the paper, and M.D.S. performed the experiments and analysed the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2005 kb)
Rights and permissions
About this article
Cite this article
Symes, M., Cronin, L. Decoupling hydrogen and oxygen evolution during electrolytic water splitting using an electron-coupled-proton buffer. Nature Chem 5, 403–409 (2013). https://doi.org/10.1038/nchem.1621
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1621
This article is cited by
-
Decoupled electrolysis for hydrogen production and hydrazine oxidation via high-capacity and stable pre-protonated vanadium hexacyanoferrate
Nature Communications (2024)
-
Electrochemical and chemical cycle for high-efficiency decoupled water splitting in a near-neutral electrolyte
Nature Materials (2024)
-
A hybrid fuel cell for water purification and simultaneously electricity generation
Frontiers of Environmental Science & Engineering (2023)
-
Recent Advances in High-Efficiency Electrocatalytic Water Splitting Systems
Electrochemical Energy Reviews (2023)
-
A photosensitizer–polyoxometalate dyad that enables the decoupling of light and dark reactions for delayed on-demand solar hydrogen production
Nature Chemistry (2022)