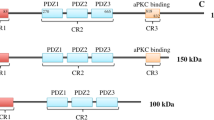
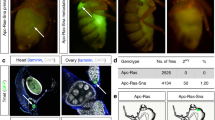
Abstract
The development of cancer is a multistep process in which the DNA of a single cell accumulates mutations in genes that control essential cellular processes. Loss of cell–cell adhesion and cell polarity is commonly observed in advanced tumours and correlates well with their invasion into adjacent tissues and the formation of metastases. Growing evidence indicates that loss of cell–cell adhesion and cell polarity may also be important in early stages of cancer. The strongest hints in this direction come from studies on tumour suppressor genes in the fruitfly Drosophila melanogaster, which have revealed their importance in the control of apical–basal cell polarity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Huber, M. A., Kraut, N. & Beug, H. Molecular requirements for epithelial–mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 17, 548–558 (2005).
Thiery, J. P. & Sleeman, J. P. Complex networks orchestrate epithelial–mesenchymal transitions. Nature Rev. Mol. Cell Biol. 7, 131–142 (2006).
Knust, E. & Bossinger, O. Composition and formation of intercellular junctions in epithelial cells. Science 298, 1955–1959 (2002).
Halbleib, J. M. & Nelson, W. J. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 20, 3199–3214 (2006).
Cavallaro, U. & Christofori, G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nature Rev. Cancer 4, 118–132 (2004).
Cowin, P., Rowlands, T. M. & Hatsell, S. J. Cadherins and catenins in breast cancer. Curr. Opin. Cell Biol. 17, 499–508 (2005).
Hermiston, M. L. & Gordon, J. I. In vivo analysis of cadherin function in the mouse intestinal epithelium: essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. J. Cell Biol. 129, 489–506 (1995).
Hermiston, M. L. & Gordon, J. I. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science 270, 1203–1207 (1995).
Graziano, F., Humar, B. & Guilford, P. The role of the E-cadherin gene (CDH1) in diffuse gastric cancer susceptibility: from the laboratory to clinical practice. Ann. Oncol. 14, 1705–1713 (2003).
Herzig, M., Savarese, F., Novatchkova, M., Semb, H. & Christofori, G. Tumor progression induced by the loss of E-cadherin independent of β-catenin/Tcf-mediated Wnt signaling. Oncogene 26, 2290–2298 (2007).
Perl, A. K., Wilgenbus, P., Dahl, U., Semb, H. & Christofori, G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 392, 190–193 (1998).
Conacci-Sorrell, M., Zhurinsky, J. & Ben-Ze'ev, A. The cadherin–catenin adhesion system in signaling and cancer. J. Clin. Invest. 109, 987–991 (2002).
Bilder, D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 18, 1909–1925 (2004).
Humbert, P., Russell, S. & Richardson, H. Dlg, Scribble and Lgl in cell polarity, cell proliferation and cancer. BioEssays 25, 542–553 (2003).
Gateff, E. Malignant neoplasms of genetic origin in Drosophila melanogaster. Science 200, 1448–1459 (1978).
Bilder, D., Li, M. & Perrimon, N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289, 113–116 (2000).
Bilder, D. & Perrimon, N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403, 676–680 (2000).
Bilder, D., Schober, M. & Perrimon, N. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nature Cell Biol. 5, 53–58 (2003).
Johnson, K. & Wodarz, A. A genetic hierarchy controlling cell polarity. Nature Cell Biol. 5, 12–14 (2003).
Tanentzapf, G. & Tepass, U. Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nature Cell Biol. 5, 46–52 (2003).
Hough, C. D., Woods, D. F., Park, S. & Bryant, P. J. Organizing a functional junctional complex requires specific domains of the Drosophila MAGUK Discs large. Genes Dev. 11, 3242–3253 (1997).
Zeitler, J., Hsu, C. P., Dionne, H. & Bilder, D. Domains controlling cell polarity and proliferation in the Drosophila tumor suppressor Scribble. J. Cell Biol. 167, 1137–1146 (2004).
Dow, L. E. et al. hScrib is a functional homologue of the Drosophila tumour suppressor Scribble. Oncogene 22, 9225–9230 (2003).
Grifoni, D. et al. The human protein Hugl-1 substitutes for Drosophila lethal giant larvae tumour suppressor function in vivo. Oncogene 23, 8688–8694 (2004).
Thomas, U., Phannavong, B., Muller, B., Garner, C. C. & Gundelfinger, E. D. Functional expression of rat synapse-associated proteins SAP97 and SAP102 in Drosophila dlg-1 mutants: effects on tumor suppression and synaptic bouton structure. Mech. Dev. 62, 161–174 (1997).
Klezovitch, O., Fernandez, T. E., Tapscott, S. J. & Vasioukhin, V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 18, 559–571 (2004).
Yamanaka, T. et al. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr. Biol. 13, 734–743 (2003).
Plant, P. J. et al. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nature Cell Biol. 5, 301–308 (2003).
Qin, Y., Capaldo, C., Gumbiner, B. M. & Macara, I. G. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J. Cell Biol. 171, 1061–1071 (2005).
Gangar, A., Rossi, G., Andreeva, A., Hales, R. & Brennwald, P. Structurally conserved interaction of Lgl family with SNAREs is critical to their cellular function. Curr. Biol. 15, 1136–1142 (2005).
Zhang, X. et al. Lethal giant larvae proteins interact with the exocyst complex and are involved in polarized exocytosis. J. Cell Biol. 170, 273–283 (2005).
Nelson, W. J. & Yeaman, C. Protein trafficking in the exocytic pathway of polarized epithelial cells. Trends Cell Biol. 11, 483–486 (2001).
Giebel, B. & Wodarz, A. Tumor suppressors: control of signaling by endocytosis. Curr. Biol. 16, R91–R92 (2006).
Gardiol, D., Zacchi, A., Petrera, F., Stanta, G. & Banks, L. Human discs large and scrib are localized at the same regions in colon mucosa and changes in their expression patterns are correlated with loss of tissue architecture during malignant progression. Int. J. Cancer 119, 1285–1290 (2006).
Kuphal, S. et al. Expression of Hugl-1 is strongly reduced in malignant melanoma. Oncogene 25, 103–110 (2006).
Nakagawa, S. et al. Analysis of the expression and localisation of a LAP protein, human scribble, in the normal and neoplastic epithelium of uterine cervix. Br. J. Cancer 90, 194–199 (2004).
Schimanski, C. C. et al. Reduced expression of Hugl-1, the human homologue of Drosophila tumour suppressor gene lgl, contributes to progression of colorectal cancer. Oncogene 24, 3100–3109 (2005).
Cavatorta, A. L. et al. Differential expression of the human homologue of Drosophila discs large oncosuppressor in histologic samples from human papillomavirus-associated lesions as a marker for progression to malignancy. Int. J. Cancer 111, 373–380 (2004).
Stoll, M. et al. Genetic variation in DLG5 is associated with inflammatory bowel disease. Nature Genet. 36, 476–480 (2004).
Massimi, P., Gammoh, N., Thomas, M. & Banks, L. HPV E6 specifically targets different cellular pools of its PDZ domain-containing tumour suppressor substrates for proteasome-mediated degradation. Oncogene 23, 8033–8039 (2004).
Massimi, P., Narayan, N., Cuenda, A. & Banks, L. Phosphorylation of the discs large tumour suppressor protein controls its membrane localisation and enhances its susceptibility to HPV E6-induced degradation. Oncogene 25, 4276–4285 (2006).
Thomas, M., Massimi, P., Navarro, C., Borg, J. P. & Banks, L. The hScrib/Dlg apico-basal control complex is differentially targeted by HPV-16 and HPV-18 E6 proteins. Oncogene 24, 6222–6230 (2005).
Brumby, A. M. & Richardson, H. E. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 22, 5769–5779 (2003).
Pagliarini, R. A. & Xu, T. A genetic screen in Drosophila for metastatic behavior. Science 302, 1227–1231 (2003).
Igaki, T., Pagliarini, R. A. & Xu, T. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr. Biol. 16, 1139–1146 (2006).
Uhlirova, M., Jasper, H. & Bohmann, D. Non-cell-autonomous induction of tissue overgrowth by JNK/Ras cooperation in a Drosophila tumor model. Proc. Natl Acad. Sci. USA 102, 13123–13128 (2005).
Uhlirova, M. & Bohmann, D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 25, 5294–5304 (2006).
Suzuki, A. & Ohno, S. The PAR-aPKC system: lessons in polarity. J. Cell Sci. 119, 979–987 (2006).
Wodarz, A. Establishing cell polarity in development. Nature Cell Biol. 4, E39–E44. (2002).
Eder, A. M. et al. Atypical PKCι contributes to poor prognosis through loss of apical–basal polarity and cyclin E overexpression in ovarian cancer. Proc. Natl Acad. Sci. USA 102, 12519–12524 (2005).
Murray, N. R. et al. Protein kinase Cι is required for Ras transformation and colon carcinogenesis in vivo. J. Cell Biol. 164, 797–802 (2004).
Regala, R. P. et al. Atypical protein kinase Cι plays a critical role in human lung cancer cell growth and tumorigenicity. J. Biol. Chem. 280, 31109–31115 (2005).
Regala, R. P. et al. Atypical protein kinase C ι is an oncogene in human non-small cell lung cancer. Cancer Res. 65, 8905–8911 (2005).
Schermer, B. et al. The von Hippel–Lindau tumor suppressor protein controls ciliogenesis by orienting microtubule growth. J. Cell Biol. 175, 547–554 (2006).
Barry, R. E. & Krek, W. The von Hippel–Lindau tumour suppressor: a multi-faceted inhibitor of tumourigenesis. Trends Mol. Med. 10, 466–472 (2004).
Okuda, H. et al. The von Hippel–Lindau tumor suppressor protein mediates ubiquitination of activated atypical protein kinase C. J. Biol. Chem. 276, 43611–43617 (2001).
Calzada, M. J. et al. von Hippel–Lindau tumor suppressor protein regulates the assembly of intercellular junctions in renal cancer cells through hypoxia-inducible factor-independent mechanisms. Cancer Res. 66, 1553–1560 (2006).
Cully, M., You, H., Levine, A. J. & Mak, T. W. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nature Rev. Cancer 6, 184–192 (2006).
Martin-Belmonte, F. et al. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128, 383–397 (2007).
Pinal, N. et al. Regulated and polarized PtdIns(3,4,5)P3 accumulation is essential for apical membrane morphogenesis in photoreceptor epithelial cells. Curr. Biol. 16, 140–149 (2006).
von Stein, W., Ramrath, A., Grimm, A., Muller-Borg, M. & Wodarz, A. Direct association of Bazooka/PAR-3 with the lipid phosphatase PTEN reveals a link between the PAR/aPKC complex and phosphoinositide signaling. Development 132, 1675–1686 (2005).
Martin, S. G. & St Johnston, D. A role for Drosophila LKB1 in anterior–posterior axis formation and epithelial polarity. Nature 421, 379–384 (2003).
Watts, J. L., Morton, D. G., Bestman, J. & Kemphues, K. J. The C. elegans par-4 gene encodes a putative serine-threonine kinase required for establishing embryonic asymmetry. Development 127, 1467–1475 (2000).
Alessi, D. R., Sakamoto, K. & Bayascas, J. R. LKB1-dependent signaling pathways. Annu. Rev. Biochem. 75, 137–163 (2006).
Baas, A. F., Smit, L. & Clevers, H. LKB1 tumor suppressor protein: PARtaker in cell polarity. Trends Cell Biol. 14, 312–319 (2004).
Baas, A. F. et al. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell 116, 457–466 (2004).
Forcet, C. et al. Functional analysis of Peutz–Jeghers mutations reveals that the LKB1 C-terminal region exerts a crucial role in regulating both the AMPK pathway and the cell polarity. Hum. Mol. Genet. 14, 1283–1292 (2005).
Lee, J. H. et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature 447, 1017–1020 (2007).
Mirouse, V., Swick, L. L., Kazgan, N., St Johnston, D. & Brenman, J. E. LKB1 and AMPK maintain epithelial cell polarity under energetic stress. J. Cell Biol. 177, 387–392 (2007).
Linggi, B. & Carpenter, G. ErbB receptors: new insights on mechanisms and biology. Trends Cell Biol. 16, 649–656 (2006).
Aranda, V. et al. Par6–aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nature Cell Biol. 8, 1235–1245 (2006).
Debnath, J. et al. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell 111, 29–40 (2002).
Zahir, N. & Weaver, V. M. Death in the third dimension: apoptosis regulation and tissue architecture. Curr. Opin. Genet. Dev. 14, 71–80 (2004).
Guo, W. et al. β4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell 126, 489–502 (2006).
Guo, W. & Giancotti, F. G. Integrin signalling during tumour progression. Nature Rev. Mol. Cell Biol. 5, 816–826 (2004).
Siegel, P. M. & Massagué, J. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nature Rev. Cancer 3, 807–821 (2003).
Derynck, R. & Zhang, Y. E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425, 577–584 (2003).
Ozdamar, B. et al. Regulation of the polarity protein Par6 by TGFβ receptors controls epithelial cell plasticity. Science 307, 1603–1609 (2005).
Al-Hajj, M., Becker, M. W., Wicha, M., Weissman, I. & Clarke, M. F. Therapeutic implications of cancer stem cells. Curr. Opin. Genet. Dev. 14, 43–47 (2004).
Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 (2001).
Al-Hajj, M. & Clarke, M. F. Self-renewal and solid tumor stem cells. Oncogene 23, 7274–7282 (2004).
Singh, S. K. et al. Identification of human brain tumour initiating cells. Nature 432, 396–401 (2004).
Pardal, R., Clarke, M. F. & Morrison, S. J. Applying the principles of stem-cell biology to cancer. Nature Rev. Cancer 3, 895–902 (2003).
Betschinger, J. & Knoblich, J. A. Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr. Biol. 14, R674–R685 (2004).
Wodarz, A. & Huttner, W. B. Asymmetric cell division during neurogenesis in Drosophila and vertebrates. Mech. Dev. 120, 1297–1309 (2003).
Wodarz, A. Molecular control of cell polarity and asymmetric cell division in Drosophila neuroblasts. Curr. Opin. Cell Biol. 17, 475–481 (2005).
Bello, B., Reichert, H. & Hirth, F. The brain tumor gene negatively regulates neural progenitor cell proliferation in the larval central brain of Drosophila. Development 133, 2639–2648 (2006).
Betschinger, J., Mechtler, K. & Knoblich, J. A. Asymmetric segregation of the tumor suppressor brat regulates self-renewal in Drosophila neural stem cells. Cell 124, 1241–1253 (2006).
Caussinus, E. & Gonzalez, C. Induction of tumor growth by altered stem-cell asymmetric division in Drosophila melanogaster. Nature Genet. 37, 1125–1129 (2005).
Lee, C. Y., Wilkinson, B. D., Siegrist, S. E., Wharton, R. P. & Doe, C. Q. Brat is a Miranda cargo protein that promotes neuronal differentiation and inhibits neuroblast self-renewal. Dev. Cell 10, 441–449 (2006).
Choksi, S. P. et al. Prospero acts as a binary switch between self-renewal and differentiation in Drosophila neural stem cells. Dev. Cell 11, 775–789 (2006).
Li, L. & Vaessin, H. Pan-neural Prospero terminates cell proliferation during Drosophila neurogenesis. Genes Dev. 14, 147–151 (2000).
Bowman, S. K., Neumuller, R. A., Novatchkova, M., Du, Q. & Knoblich, J. A. The Drosophila NuMA homolog Mud regulates spindle orientation in asymmetric cell division. Dev. Cell 10, 731–742 (2006).
Izumi, Y., Ohta, N., Hisata, K., Raabe, T. & Matsuzaki, F. Drosophila Pins-binding protein Mud regulates spindle-polarity coupling and centrosome organization. Nature Cell Biol. 8, 586–593 (2006).
Lee, C. Y. et al. Drosophila Aurora-A kinase inhibits neuroblast self-renewal by regulating aPKC/Numb cortical polarity and spindle orientation. Genes Dev. 20, 3464–3474 (2006).
Siller, K. H., Cabernard, C. & Doe, C. Q. The NuMA-related Mud protein binds Pins and regulates spindle orientation in Drosophila neuroblasts. Nature Cell Biol. 8, 594–600 (2006).
Wang, H. et al. Aurora-A acts as a tumor suppressor and regulates self-renewal of Drosophila neuroblasts. Genes Dev. 20, 3453–3463 (2006).
Du, Q. & Macara, I. G. Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell 119, 503–516 (2004).
Lee, C. Y., Robinson, K. J. & Doe, C. Q. Lgl, Pins and aPKC regulate neuroblast self-renewal versus differentiation. Nature 439, 594–598 (2006).
Rolls, M. M., Albertson, R., Shih, H. P., Lee, C. Y. & Doe, C. Q. Drosophila aPKC regulates cell polarity and cell proliferation in neuroblasts and epithelia. J. Cell Biol. 163, 1089–1098 (2003).
Giet, R., Petretti, C. & Prigent, C. Aurora kinases, aneuploidy and cancer, a coincidence or a real link? Trends Cell Biol. 15, 241–250 (2005).
Wang, X. et al. Overexpression of aurora kinase A in mouse mammary epithelium induces genetic instability preceding mammary tumor formation. Oncogene 25, 7148–7158 (2006).
Lechler, T. & Fuchs, E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437, 275–280 (2005).
Cairns, J. Mutation selection and the natural history of cancer. Nature 255, 197–200 (1975).
Potten, C. S., Owen, G. & Booth, D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J. Cell Sci. 115, 2381–2388 (2002).
Yamashita, Y. M., Jones, D. L. & Fuller, M. T. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science 301, 1547–1550 (2003).
Acknowledgements
We thank Claudia Binder, Olaf Bossinger and Tobias Pukrop for critical comments on the manuscript. Work in our laboratories is funded by grants from the Deutsche Forschungsgemeinschaft (DFG Research Center for Molecular Physiology of the Brain (CMPB), Sonderforschungsbereich 523) to A.W. and by Cancer Research UK to I.N.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wodarz, A., Näthke, I. Cell polarity in development and cancer. Nat Cell Biol 9, 1016–1024 (2007). https://doi.org/10.1038/ncb433
Issue Date:
DOI: https://doi.org/10.1038/ncb433
This article is cited by
-
Therapeutic Effectiveness of Anticancer Agents Targeting Different Signaling Molecules Involved in Asymmetric Division of Cancer Stem Cell
Stem Cell Reviews and Reports (2023)
-
AVL9 promotes colorectal carcinoma cell migration via regulating EGFR expression
Biological Procedures Online (2022)
-
DGKZ promotes TGFβ signaling pathway and metastasis in triple-negative breast cancer by suppressing lipid raft-dependent endocytosis of TGFβR2
Cell Death & Disease (2022)
-
Lack of extracellular matrix switches TGF-β induced apoptosis of endometrial cells to epithelial to mesenchymal transition
Scientific Reports (2022)
-
Myosin Vb as a tumor suppressor gene in intestinal cancer
Oncogene (2022)