Abstract
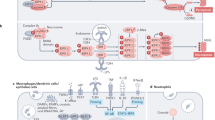
Nuclear DNA damage and ligation of plasma-membrane death receptors have long been recognized as initial triggers of apoptosis that induce mitochondrial membrane permeabilization (MMP) and/or the direct activation of caspases. Accumulating evidence suggests that other organelles, including the endoplasmic reticulum (ER), lysosomes and the Golgi apparatus, are also major points of integration of pro-apoptotic signalling or damage sensing. Each organelle possesses sensors that detect specific alterations, locally activates signal transduction pathways and emits signals that ensure inter-organellar cross-talk. The ER senses local stress through chaperones, Ca2+-binding proteins and Ca2+ release channels, which might transmit ER Ca2+ responses to mitochondria. The ER also contains several Bcl-2-binding proteins, and Bcl-2 has been reported to exert part of its cytoprotective effect within the ER. Upon membrane destabilization, lysosomes release cathepsins that are endowed with the capacity of triggering MMP. The Golgi apparatus constitutes a privileged site for the generation of the pro-apoptotic mediator ganglioside GD3, facilitates local caspase-2 activation and might serve as a storage organelle for latent death receptors. Intriguingly, most organelle-specific death responses finally lead to either MMP or caspase activation, both of which might function as central integrators of the death pathway, thereby streamlining lysosome-, Golgi- or ER-elicited responses into a common pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kerr, J. F. R., Wyllie, A. H. & Currie, A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26, 239–257 (1972).
Nicholson, D. W. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 6, 1028–1042 (1999).
Kroemer, G. & Reed, J. C. Mitochondrial control of cell death. Nature Med. 6, 513–519 (2000).
Bursch, W. The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ. 8, 569–581 (2001).
Green, D. R. & Kroemer, G. The central executioner of apoptosis: mitochondria or caspases? Trends Cell Biol. 8, 267–271 (1998).
Finkel, E. The mitochondrion: Is it central to apoptosis? Science 292, 624–626 (2001).
Kirsch, D. G. et al. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J. Biol. Chem. 274, 21155–21161 (1999).
Condorelli, F. et al. Caspase cleavage enhances the apoptosis-inducing effects of BAD. Mol. Cell. Biol. 21, 3025–3036 (2001).
Korsmeyer, S. J. et al. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 7, 1166–1173 (2000).
Susin, S. A. et al. Two distinct pathways leading to nuclear apoptosis. J. Exp. Med. 192, 571–579 (2000).
Joza, N. et al. Essential role of the mitochondrial apoptosis inducing factor in programmed cell death. Nature 410, 549–554 (2001).
Kroemer, G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nature Med. 3, 614–620 (1997).
Martinou, J.-C. & Green, D. R. Breaking the mitochondrial barrier. Nature Rev. Mol. Cell Biol. 2, 63–67 (2001).
Zamzami, N. & Kroemer, G. Mitochondria in apoptosis. How Pandora's box opens. Nature Rev. Mol. Cell Biol. 2, 67–71 (2001).
Vieira, H. L. et al. Mitochondrial membrane permeabilization during apoptosis. Impact of the adenine nucleotide translocator. Cell Death Differ. 7, 1146–1154 (2000).
Costantini, P., Jacotot, E., Decaudin, D. & Kroemer, G. Mitochondrion as a novel target of anti-cancer chemotherapy. J. Natl Cancer Inst. 92, 1042–1053 (2000).
Rodrigues, C. M., Sola, S., Silva, R. & Brites, D. Bilirubin and amyloid-β peptide induce cytochrome c release through mitochondrial membrane permeabilization. Mol. Med. 6, 936–946 (2000).
Botla, R., Spivey, J. R., Aguilar, H., Bronk, S. G. & Gores, G. J. Ursodeoxycholate (UDCA) inhibits the mitochondrial permeability transition induced by glycochenodeoxycholate: a mechanism of UDCA cytoprotection. J. Pharmacol. Exp. Ther. 272, 930–938 (1995).
Parks, J. K., Smith, T. S., Trimmer, P. A., Bennett, J. P. J. & Parker, W. D. J. Neurotoxic Aβ peptides increase oxidative stress in vivo through NMDA receptor and nitric-oxide-synthase mechanisms, and inhibit complex IV activity and induce a mitochondrial permeability transition in vitro. J. Neurochem. 76, 1050–1056 (2001).
Boya, P., Roques, B. & Kroemer, G. (2001). Bacterial and viral proteins regulating apoptosis at the mitochondrial level. EMBO J. (in the press).
Zheng, T. S. Death by design: the big debut of small molecules. Nature Cell Biol. 3, E1–E3 (2001).
Wong, A. et al. The Friedreich's ataxia mutation confers cellular sensitivity to oxidant stress which is rescued by chelators of iron and caclium and inhibitors of apoptosis. Hum. Mol. Genet. 8, 425–430 (1999).
Wang, J., Silva, J. P., Gustafsson, C. M., Rustin, P. & Larsson, N. G. Increased in vivo apoptosis in cells lacking mitochondrial DNA gene expression. Proc. Natl Acad. Sci. USA 98, 4038–4043 (2001).
Fliss, M. S. et al. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science 287, 2017–2019 (2000).
Singh, K. K. et al. Mitochondrial DNA determines the cellular response to cancer therapeutic agents. Oncogene 18, 6641–6646 (1999).
Zhou, B.-B. S. & Elledge, S. J. The DNA damage response: putting checkpoints in perspective. Nature 408, 433–439 (2000).
Rich, T., Allen, R. L. & Wyllie, A. H. Defying death after DNA damage. Nature Cell Biol. 407, 777–783 (2000).
Vogelstein, B., Lane, D. & Levine, A. J. Surfing the p53 network. Nature 16, 307–310 (2000).
Oda, E. et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288, 1053–1058 (2000).
Yu, J., Zhang, L., Hwang, P. M., Kinzler, J. W. & Vogelstein, B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol. Cell 7, 673–682 (2001).
Deng, Y. & Wu, X. Peg3/Pw1 promotes p53-mediated apoptosis by inducing bax translocation from cytosol to mitochondria. Proc. Natl Acad. Sci. USA 97, 12050–12055 (2000).
Donald, S. P. et al. Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res. 61, 1810–1815 (2001).
Oda, K. et al. p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell 102, 849–862 (2000).
Moroni, M. C. et al. Apaf-1 is a transcriptional target for E2F and p53. Nature Cell Biol. 3, 552–558 (2001).
Marchenko, N. D., Zaika, A. & Moll, U. M. Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J. Biol. Chem. 275, 16202–16212 (2000).
Kumar, S. et al. Targeting of the c-Abl tyrosine kinase to mitochondria in the necrotic cell death response to oxidative stress. J. Biol. Chem. 276, 17281–17285 (2001).
Taneja, N., Tjalkens, R., Philbert, M. A. & Rehemtulla, A. Irradiation of mitochondria initiates apoptosis in a cell free system. Oncogene 20, 167–177 (2001).
Leach, J. K., Van Tuyle, G., Lin, P. S., Schmidt-Ullrich, R. & Mikkelsen, R. B. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 61, 3894–3901 (2001).
Kaufman, R. J. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 13, 1211–1233 (1999).
Patil, C. & Walter, P. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr. Opin. Cell Biol. 13, 349–356 (2001).
Katayama, T. et al. Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nature Cell Biol. 1, 479–485 (1999).
Iwawaki, T. et al. Translational control by the ER transmembrane kinase/ribonuclease IRE1 under ER stress. Nature Cell Biol. 158–164 (2001).
Urano, F. et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287, 664–666 (2000).
Niwa, M., Sidrauski, C., Kaufman, R. J. & Walter, P. A role for presenilin-1 in nuclear accumulation of Ire1 fragments and induction of the mammalian unfolded response. Cell 99, 691–702 (1999).
McCullough, K. D., Martindale, J. L., Klotz, L. O., Aw, T. Y. & Holbrook, N. J. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulation Bcl-2 and perturbing the cellular redox state. Mol. Cell. Biol. 21, 1249–1259 (2001).
Zinszner, H. et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 12, 982–995 (1998).
Nakagawa, T. et al. Caspase-12 mediates endoplasmic reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 403, 98–103 (2000).
Nakagawa, T. & Yuan, J. Cross-talk between two cysteine protease families: activation of caspase-12 by calpain in apoptosis. J. Cell Biol. 150, 887–894 (2000).
Yoneda, T. et al. Activation of caspase-12, an endoplasmic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanisms in response to ER stress. J. Biol. Chem. 276, 13935–13940 (2001).
Berridge, M. J., Lipp, P. & Bootman, M. D. The versatility and universality of calcium signalling. Nature Rev. Mol. Cell Biol. 1, 11–21 (2000).
Jayaraman, T. & Marks, A. R. T cells deficient in inositol 1,4,5-trisphosphate receptor are resistant to apoptosis. Mol. Cell. Biol. 17, 3005–3012 (1997).
Khan, A. A. et al. Lymphocyte apoptosis: mediation by increased type 3 inositol 1,4,5-trisphosphate receptor. Science 273, 503–507 (1996).
Blackshaw, S. et al. Type 3 inositol 1,4,5,-trisphosphate receptor modulates cell death. FASEB J. 14, 1375–1379 (2000).
Miyake, H., Hara, I., Arakawa, S. & Kamidono, S. Stress protein GRP78 prevents apoptosis induced by calcium ionophore, ionomycin, but not by glycosylation inhibitor, tunicamycin, in human prostate cancer cells. J. Cell. Biochem. 77, 396–408 (2000).
Jamora, C., Dennert, G. & Lee, A. S. Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proc. Natl Acad. Sci. USA 93, 7690–7694 (1996).
Johnson, S., Michalak, M., Opas, M. & Eggleton, P. The ins and outs of calreticulin: from the ER lumen to the extracellular space. Trends Cell Biol. 11, 122–129 (2001).
Szalai, G., Krischnamurthy, R. & Hajnoczky, G. Apoptosis driven by IP3-linked mitochondrial calcium signals. EMBO J. 18, 6349–6361 (1999).
Nakamura, K. et al. Changes in the endoplasmic reticulum luminal environment affect cell sensitivity to apoptosis. J. Cell Biol. 150, 731–740 (2000).
Wei, M. C. et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730 (2001).
Tagami, S., Eguchi, Y., Kinoshita, M., Takeda, M. & Tsujimoto, Y. A novel protein, RTN-XS, interacts with both Bcl-XL and Bcl-2 on endoplasmic reticulum and reduces their anti-apoptotic activity. Oncogene 19, 5736–5746 (2000).
Hacki, J. et al. Apoptotic crosstalk between the endoplasmic reticulum and mitochondria controlled by Bcl-2. Oncogene 19, 2286–2295 (2000).
Annis, M. G. et al. Endoplasmic reticulum localized Bcl-2 prevents apoptosis when redistribution of cytochrome c is a late event. Oncogene 20, 1939–1952 (2001).
Pinton, P. et al. Reduced loading of intracellular Ca2+ stores and downregulation of capacitative Ca2+ flux in Bcl-2-overexpressing cells. J. Cell Biol. 148, 857–862 (2001).
Foyouzi-Youssefi, R. et al. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc. Natl Acad. Sci. USA 97, 5723–5728 (2000).
Pinton, P. et al. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 20, 2690–2701 (2001).
Chami, M. et al. SERCA1 truncated proteins unable to pump calcium reduce the endoplamic reticulum calcium concentration and induce apoptosis. J. Cell Biol. 153, 1301–1313 (2001).
Distelhorst, C. W., Lam, M. & McCormick, T. S. Bcl-2 inhibits hydrogen peroxide-induced ER Ca2+ pool depletion. Oncogene 12, 2051–2055 (1996).
He, H. L., Lam, M., McCormick, T. S. & Distelhorst, C. W. Maintenance of calcium homeostasis in the endoplasmic reticulum by Bcl-2. J. Cell Biol. 138, 1219–1228 (1997).
Pan, Z., Damron, D., Nieminen, A. M., Bhat, M. B. & Ma, J. Depeletion of intracellular Ca2+ by caffeine and ryanodine induces apoptosis of chinese hamster ovary cells transfected with ryanodine receptor. J. Biol. Chem. 275, 19978–19984 (2000).
Mattson, M. P., Zhu, H., Yu, J. & Kindy, M. S. Presenilin-1 mutation increases neuronal vulnerability to focal ischemia in vivo and to hypoxia and glucose deprivation in cell culture: involvement of perturbed calcium homeostasis. J. Neurosci. 20, 1358–1364 (2000).
Schneider, I. et al. Mutant presenilins disturb neuronal calcium homeostasis in the brain of transgenic mice, decreasing the threshold for excitotoxicity and facilitating long-term potentiation. J. Biol. Chem. 276, 11539–11544 (2001).
Mancini, M. et al. Caspase-2 is localized at the Golgi complex and cleaves golgin-160 during apoptosis. J. Cell Biol. 149, 603–612 (2000).
Bennett, M. et al. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science 282, 290–293 (1998).
Zhang, X. D., Fanco, A. V., Nguyen, T., Gray, C. P. & Hersey, P. Differential localization and regulation of death and decoy receptors for TNF-related apoptosis-inducing ligand (TRAIL) in human melanoma cells. J. Immunol. 164, 3961–3970 (2000).
DeMaria, R. et al. Requirement for GD3 ganglioside in CD95- and ceramide-induced apoptosis. Science 277, 1652–1655 (1997).
Rippo, M. R. et al. Ganglioside GD3 directly targets mitochondria in a Bcl-2 controlled fashion. FASEB J. 14, 2047–2054 (2000).
Goss, P. E., Baker, M. A., Carver, J. P. & Dennis, J. W. Inhibitors of carbohydrate processing: a new class of anticancer agents. Clin. Cancer Res. 1, 935–944 (1995).
Prendergast, G. C. Farnesyltransferase inhibitors: antineoplastic mechanisms and clinical prospects. Curr. Opin. Cell Biol. 12, 166–173 (2000).
Liu, A.-X., Cerniglia, G. J., Berhard, E. J. & Prendergast, G. C. RhoB is required to mediate apoptosis in neoplastically transformed cells after DNA damage. Proc. Natl Acad. Sci. USA 98, 6192–6197 (2001).
Ferri, K. F. & Kroemer, G. Control of apoptotic DNA degradation. Nature Cell Biol. 2, E63–E64 (2001).
Li, W. et al. Induction of cell death by the lysosomotropic detergent MSDH. FEBS Lett. 17, 35–39 (2000).
Dai, H. et al. The polyamine oxidase inhibitor MDL-75,527 selectively induces apoptosis of transformed hematopoietic cells through lysosomotropic effects. Cancer Res. 59, 4944–4954 (1999).
Zhao, M., Eaton, J. W. & Brunk, U. T. Protection against oxidant-mediated lysosomal rupture: a new anti-apoptotic activity of Bcl-2? FEBS Lett. 485, 104–108 (2000).
Deiss, L. P., Galinka, H., Berissi, H., Cohen, O. & Kimchi, A. Cathepsin D protease mediates programmed cell death induced by interferon-gamma, Fas/APO-1 and TNF-alpha. EMBO J. 15, 3861–3870 (1996).
Roberg, K. Relocalization of cathepsin D and cytochrome c early in apoptosis revealed by immunoelectron microscopy. Lab. Invest. 81, 149–158 (2001).
Heinrich, M. et al. Cathepsin D targeted by acid sphingomyelinase-derived ceramide. EMBO J. 18, 5252–5263 (1999).
Guicciardi, M. E. et al. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J. Clin. Invest. 106, 1127–1137 (2000).
Foghsgaard, M. et al. Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J. Cell Biol. 153, 999–1009 (2001).
Schotte, P. et al. Cathepsin B-mediated activation of the proinflammatory caspase-11. Biochem. Biophys. Res. Commun. 251, 379–387 (1998).
Stoka, V. et al. Lyosomal protease pathways to apoptosis: cleavage of bid, not pro-caspase, is the most likely route. J. Biol. Chem. 276, 3149–3157 (2001).
Suter, M. et al. Age-related macular degeneration. The lipofuscin component N-retinyl-N-retinylidene ethanolamine detaches proapoptotic proteins from mitochondria and induces apoptosis in mammalian retinal pigment cells. J. Biol. Chem. 275, 39625–39630 (2000).
Farina, F. et al. Involvement of caspase-3 and GD3 ganglioside in ceramide-induced apoptosis in Farber disease. J. Histochem. Cytochem. 48, 57–62 (2000).
Zhang, Z. et al. Lysosomal ceroid depletion by drugs: Therapeutic implications for a hereditary neurodegenerative disease of childhood. Nature Med. 7, 478–484 (2001).
Puraman, K. L., Guo, W. X., Quian, W. H., Nikbakht, K. & Boustany, R. M. CLN3 defines a novel antiapoptotic pathway operative in neurodegeneration and mediated by ceramide. Mol. Genet. Metab. 66, 294–308 (1999).
Pennacchio, L. A. et al. Mutations in the gene encoding cystatin B in progressive myoclonus epilepsy (EPM1). Science 271, 1731–1734 (1996).
Pennacchio, L. A. et al. Progressive ataxia, myoclonic epilepsy and cerebellar apoptosis in cystatin B-deficient mice. Nature Genet. 20, 251–258 (1998).
Wang, Z.-G. et al. Pml is essential for multiple apoptotic pathways. Nature Genet. 20, 266–272 (1998).
Bottero, V. et al. IκB α, the NF-κB inhibitory subunit, interacts with ANT, the mitochondrial ATP/ADP translocator. J. Biol. Chem. (in the press).
Beere, H. M. & Green, D. R. Stress management — heat shock protein-70 and the regulation of apoptosis. Trends Cell Biol. 11, 6–10 (2001).
Ravagnan, L. et al. Heat shock protein 70 antagonizes apoptosis inducing factor. Nature Cell Biol. (in the press).
Xue, L., Fletcher, G. C. & Tolkovsky, A. M. Mitochondria are selectively eliminated from eukaryotic cells after blockade of caspases during apoptosis. Curr. Biol. 6, 361–365 (2000).
Lemasters, J. J. et al. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim. Biophys. Acta 1366, 177–196 (1998).
Brewer, J. W. & Diehl, J. A. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc. Natl Acad. Sci. USA 97, 12625–12630 (2000).
Acknowledgements
This work was supported by a special grant from the Ligue Nationale contre le Cancer, as well as grants from ANRS, FRM and the European Commission (QLG1-1999-00739 to G.K.). K.F.F. is in receipt of a fellowship from the French Ministry of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferri, K., Kroemer, G. Organelle-specific initiation of cell death pathways. Nat Cell Biol 3, E255–E263 (2001). https://doi.org/10.1038/ncb1101-e255
Issue Date:
DOI: https://doi.org/10.1038/ncb1101-e255
This article is cited by
-
RIPK1 inhibition contributes to lysosomal membrane stabilization in ischemic astrocytes via a lysosomal Hsp70.1B-dependent mechanism
Acta Pharmacologica Sinica (2023)
-
Research progress on the mechanism of curcumin in cerebral ischemia/reperfusion injury: a narrative review
Apoptosis (2023)
-
An endoplasmic reticulum stress-related signature could robustly predict prognosis and closely associate with response to immunotherapy in pancreatic ductal adenocarcinoma
Journal of Cancer Research and Clinical Oncology (2023)
-
Alcohol Intake Provoked Cardiomyocyte Apoptosis Via Activating Calcium-Sensing Receptor and Increasing Endoplasmic Reticulum Stress and Cytosolic [Ca2+]i
Cell Biochemistry and Biophysics (2023)
-
Phospholipid scramblase 3: a latent mediator connecting mitochondria and heavy metal apoptosis
Cell Biochemistry and Biophysics (2023)