Abstract
Stabilization of mutant p53 (mutp53) in tumours greatly contributes to malignant progression. However, little is known about the underlying mechanisms and therapeutic approaches to destabilize mutp53. Here, through high-throughput screening we identify statins, cholesterol-lowering drugs, as degradation inducers for conformational or misfolded p53 mutants with minimal effects on wild-type p53 (wtp53) and DNA contact mutants. Statins preferentially suppress mutp53-expressing cancer cell growth. Specific reduction of mevalonate-5-phosphate by statins or mevalonate kinase knockdown induces CHIP ubiquitin ligase-mediated nuclear export, ubiquitylation, and degradation of mutp53 by impairing interaction of mutp53 with DNAJA1, a Hsp40 family member. Knockdown of DNAJA1 also induces CHIP-mediated mutp53 degradation, while its overexpression antagonizes statin-induced mutp53 degradation. Our study reveals that DNAJA1 controls the fate of misfolded mutp53, provides insights into potential strategies to deplete mutp53 through the mevalonate pathway–DNAJA1 axis, and highlights the significance of p53 status in impacting statins’ efficacy on cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Lane, D. & Levine, A. p53 research: the past thirty years and the next thirty years. Cold Spring Harb. Perspect. Biol. 2, a000893 (2010).
Levav-Cohen, Y. et al. The p53-Mdm2 loop: a critical juncture of stress response. Subcell. Biochem. 85, 161–186 (2014).
Rivlin, N., Koifman, G. & Rotter, V. p53 orchestrates between normal differentiation and cancer. Semin. Cancer Biol. 32, 10–17 (2015).
Boeckler, F. M. et al. Targeted rescue of a destabilized mutant of p53 by an in silico screened drug. Proc. Natl Acad. Sci. USA 105, 10360–10365 (2008).
Terzian, T. et al. The inherent instability of mutant p53 is alleviated by Mdm2 or p16INK4a loss. Genes Dev. 22, 1337–1344 (2008).
Lukashchuk, N. & Vousden, K. H. Ubiquitination and degradation of mutant p53. Mol. Cell. Biol. 27, 8284–8295 (2007).
Alexandrova, E. M. et al. Improving survival by exploiting tumour dependence on stabilized mutant p53 for treatment. Nature 523, 352–356 (2015).
Masciarelli, S. et al. Gain-of-function mutant p53 downregulates miR-223 contributing to chemoresistance of cultured tumor cells. Oncogene 33, 1601–1608 (2014).
Rivlin, N., Brosh, R., Oren, M. & Rotter, V. Mutations in the p53 tumor suppressor gene: important milestones at the various steps of tumorigenesis. Genes Cancer 2, 466–474 (2011).
Zhong, S. et al. Statin use and mortality in cancer patients: Systematic review and meta-analysis of observational studies. Cancer Treat. Rev. 41, 554–567 (2015).
Li, M. et al. Mono- versus polyubiquitination: differential control of p53 fate by Mdm2. Science 302, 1972–1975 (2003).
Altwairgi, A. K. Statins are potential anticancerous agents (review). Oncol. Rep. 33, 1019–1039 (2015).
Shimoyama, S. Statins are logical candidates for overcoming limitations of targeting therapies on malignancy: their potential application to gastrointestinal cancers. Cancer Chemother. Pharmacol. 67, 729–739 (2011).
Freed-Pastor, W. A. et al. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell 148, 244–258 (2012).
Varley, J. M. et al. A detailed study of loss of heterozygosity on chromosome 17 in tumours from Li-Fraumeni patients carrying a mutation to the TP53 gene. Oncogene 14, 865–871 (1997).
Venkatachalam, S. et al. Retention of wild-type p53 in tumors from p53 heterozygous mice: reduction of p53 dosage can promote cancer formation. EMBO J. 17, 4657–4667 (1998).
McDonough, H. & Patterson, C. CHIP: a link between the chaperone and proteasome systems. Cell Stress Chaperones 8, 303–308 (2003).
Edkins, A. L. CHIP: a co-chaperone for degradation by the proteasome. Subcell. Biochem. 78, 219–242 (2015).
Peng, Y., Chen, L., Li, C., Lu, W. & Chen, J. Inhibition of MDM2 by hsp90 contributes to mutant p53 stabilization. J. Biol. Chem. 276, 40583–40590 (2001).
Li, D. et al. Functional inactivation of endogenous MDM2 and CHIP by HSP90 causes aberrant stabilization of mutant p53 in human cancer cells. Mol. Cancer Res. 9, 577–588 (2011).
Muller, P., Hrstka, R., Coomber, D., Lane, D. P. & Vojtesek, B. Chaperone-dependent stabilization and degradation of p53 mutants. Oncogene 27, 3371–3383 (2008).
Zhou, P. et al. ErbB2 degradation mediated by the co-chaperone protein CHIP. J. Biol. Chem. 278, 13829–13837 (2003).
Murata, S., Minami, Y., Minami, M., Chiba, T. & Tanaka, K. CHIP is a chaperone-dependent E3 ligase that ubiquitylates unfolded protein. EMBO Rep. 2, 1133–1138 (2001).
Hiraki, M. et al. Small-molecule reactivation of mutant p53 to wild-type-like p53 through the p53-Hsp40 regulatory axis. Chem. Biol. 22, 1206–1216 (2015).
King, F. W., Wawrzynow, A., Hohfeld, J. & Zylicz, M. Co-chaperones Bag-1, Hop and Hsp40 regulate Hsc70 and Hsp90 interactions with wild-type or mutant p53. EMBO J. 20, 6297–6305 (2001).
Yue, X. et al. BAG2 promotes tumorigenesis through enhancing mutant p53 protein levels and function. Elife 4, e08401 (2015).
Fan, C. Y., Lee, S. & Cyr, D. M. Mechanisms for regulation of Hsp70 function by Hsp40. Cell Stress Chaperones 8, 309–316 (2003).
Gandelman, K., Malhotra, B., LaBadie, B. B., Crownover, P. & Bergstrom, T. Analytes of interest and choice of dose: two important considerations in the design of bioequivalence studies with atorvastatin. Bioequiv. Bioavailab. 3, 62–68 (2011).
Bisgaier, C. L. et al. Attenuation of plasma low density lipoprotein cholesterol by select 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors in mice devoid of low density lipoprotein receptors. J. Lipid Res. 38, 2502–2515 (1997).
Moon, H., Hill, M. M., Roberts, M. J., Gardiner, R. A. & Brown, A. J. Statins: protectors or pretenders in prostate cancer? Trends Endocrinol. Metab. 25, 188–196 (2014).
Baandrup, L., Dehlendorff, C., Friis, S., Olsen, J. H. & Kjaer, S. K. Statin use and risk for ovarian cancer: a Danish nationwide case-control study. Br. J. Cancer 112, 157–161 (2015).
Zhang, X. L. et al. Statin use and risk of kidney cancer: a meta-analysis of observational studies and randomized trials. B. J. Clin. Pharmacol. 77, 458–465 (2014).
Qiu, X. B., Shao, Y. M., Miao, S. & Wang, L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell Mol. Life Sci. 63, 2560–2570 (2006).
Kota, P., Summers, D. W., Ren, H. Y., Cyr, D. M. & Dokholyan, N. V. Identification of a consensus motif in substrates bound by a Type I Hsp40. Proc. Natl Acad. Sci. USA 106, 11073–11078 (2009).
Acknowledgements
We thank B. Vogelstein (Johns Hopkins Medicine, USA), D. R. Welch (University of Kansas Medical Center, USA), S. Anant (University of Kansas Medical Center, USA), D. A. Dixon (University of Kansas Medical Center, USA), J. Lewis-Wambi (University of Kansas Medical Center, USA), S. Thomas (University of Kansas Medical Center, USA), T. Komiya (University of Kansas Medical Center, USA) and G. Lozano (MD Anderson Cancer Center, USA) for providing cell lines. We also thank A. K. Godwin, S. Hyter, R. Perez, N. K. Sharma, R. Pradhan, M. Danley, T. Izumi and R. Stein for technical assistance and helpful discussion. Research reported in this publication was supported by NIH R01-CA174735-01A1 (T.I.), P30-GM103495 (T.I.) and P30-CA168524-02 (T.I.) grants, and utilized the Lead Development and Optimization Shared Resource.
Author information
Authors and Affiliations
Contributions
T.I. supervised the project. A.P., A.Ranjan, S.V.I. and A.Roy performed the experiments. A.Roy performed high-throughput analyses. T.I. and A.P. wrote the manuscript. A.Ranjan, S.V.I., S.P., S.J.W. and A.Roy commented on experiments and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Screening strategy to identify statins as compounds that deplete p53R175H.
(a) Saos2 (p53null) cells were infected with a lentiviral vector encoding a chimeric fusion protein of p53R175H and luciferase reporter (p53R175H-Luc). Compounds that induce p53R175H degradation are also expected to degrade the p53R175H-Luc fusion protein, thereby reducing luciferase activities. The p53R175H-Luc cells were exposed to chemical libraries containing ∼9,000 compounds (2.5 μM, bioactives and FDA approved drugs) and DMSO (control) for 24 h (1st screening). Forty four (44) compounds showing reduced luciferase activity compared with DMSO were examined for the dose dependency in the luciferase activity using p53R175H-Luc cells, as well as for a counter screening using Saos2 cells expressing only luciferase reporter (Luc, 2nd screening). Top 10 compounds that preferentially reduced luciferase activity in p53R175H-Luc cells with dose dependency were selected. (b) Representative results of WB for p53 and vinculin using MG63 (p53null) exogenously expressing p53R175H or SK-Br-3 (p53R175H) and U2OS (p53wt) treated with 7 compounds other than statins for 24 h. (c) WB for p53 and vinculin using SK-Br-3 cells treated with DMSO (D) or lovastatin (L; 4 μM) for indicated time points (h: h). NT, not treated. (d) WB for p53 and vinculin using SJSA-1 (p53wt) cells infected with retroviral vector encoding control empty (control) or p53R175H cDNA (+ p53R175H) and treated with D or L (4 μM) for 24 h. (e) WB for p53 and vinculin using H-2087 (p53V 157F) cells treated with D or L for 24 h.
Supplementary Figure 2 Statins induce ubiquitination and degradation of mutp53 via an E3 ubiquitin ligase CHIP, but not MDM2.
(a) Quantitative RT-PCR for human p53 and GAPDH using total RNAs from SK-Br-3 and U2OS cells treated with DMSO (D) or lovastatin (L) at 4 μM for 24 h. Data are shown as relative p53 mRNA expression to that of GAPDH. Error bars, means ± s.d. (n = 3 independent experiments), NS: not significant; Student’s t test (two-tailed). (b) WB for p53 and vinculin using KHOS/NP (p53R156P) cells treated with cyclohexamide (CHX, 50 nM) for indicated time period following pre-incubation with D or 4 μM of L for 12 h (top). Graph showing relative p53R175H levels with time compared to those without CHX treatment (bottom). (c) Ubiquitination assays for p53 using KHOS/NP (left) or CAL33 (p53R175H, right) cells treated with D or 4 μM of L for 22 h. High molecular bands indicate ubiquitinated mutp53. (d) WB for indicated proteins using KHOS/NP cells infected with non-silencing control or MDM2 (M1, 2, 3) shRNA-encoding lentiviral vectors and treated with L at 4 μM for 24 h. (e) WB for indicated proteins using KHOS/NP cells infected with non-silencing control or CHIP (CH1, 2) shRNA-encoding lentiviral vectors and treated with L at 4 μM for 24 h. (f) WB for p53 and vinculin using SK-Br-3 (p53R175H) cells infected with non-silencing control or CHIP shRNA-encoding lentiviral vectors and treated with cyclohexamide (CHX, 50 nM) for indicated time period following pre-incubation with D or 4 μM of L for 12 h (left). Graph showing relative p53R175H levels with time, compared to those without CHX treatment (right). Error bars, means ± s.d. (n = 3 independent experiments). Statistics source data for a and f are provided in Supplementary Table 1.
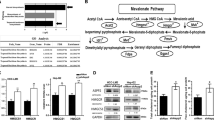
Supplementary Figure 3 Both MVP and MVA-5PP rescue cholesterol production that is inhibited by statins, whereas only MVP, but not MVA-5PP, nullifies statin-induced mutp53 degradation.
(a) WB for p53 and vinculin using KHOS/NP cells treated with DMSO (D) or lovastatin (L; 4 μM) with or without supplementation with MVA-5PP (100 and 200 μM). (b) WB for p53 and vinculin using SK-Br-3 (p53R175H) cells treated with cyclohexamide (CHX, 50 nM) for indicated time period, following pre-incubation with D, L (4 μM), L + MVP (200 μM), or L + MVA-5PP (200 μM) for 12 h (left). In Supplementary Fig. 2f and 3b, the same samples for DMSO and lovastatin treatments were used till 8 h-time points, and hence, the same data are used in both figures. Graph showing relative p53R175H levels with time compared to those without CHX treatment (right). Error bars, means ± s.d. (n = 3 independent experiments). (c) Measurement of intracellular cholesterol. SK-Br-3 (left) and KHOS/NP (right) cells were treated with L, along with MVP (200 μM) or MVA-5PP (200 μM) for 24 h, and the levels of cholesterol were determined using the Total Cholesterol Assay Kit (Colorimetric; Cell Biolabs, San Diego, CA). Graphs showing cholesterol values relative to that in D-treated cells. Error bars, means ± s.d. (n = 3 independent experiments), ∗P < 0.05, ∗∗P < 0.01; Student’s t test (two-tailed). (d) Measurement of intracellular cholesterol levels in CAL33 cells infected with non-silencing control or MVK shRNA-encoding lentiviral vectors and treated with vehicle (water), MVA (200 μM), or MVP (200 μM). Error bars, means ± s.d. (n = 3 independent experiments), ∗P < 0.05, ∗∗P < 0.01, NS: not significant; Student’s t test (two-tailed). Statistics source data for b, c, and d are provided in Supplementary Table 1.
Supplementary Figure 4 Effects of statins on oncogenic properties of human cancer cells.
(a) MTT assays using SK-Br-3, HT29 cells, as well as HCT116 sub-cell lines (p53wt, p53null, p53null+R175H, p53null+R273H) treated with different concentrations of DMSO or lovastatin. Error bars, means ± s.d. (n = 3 independent experiments), ∗P < 0.05, ∗∗P < 0.01, NS: not significant; Student’s t test (two-tailed). Results of WB for p53 in HCT116 p53wt, p53null+R175H, and p53null+R273H cells treated with DMSO or lovastatin are also shown on the right. (b) Colony formation assays using SK-Br-3, HT29, and HCT116 sub-cell lines seeded onto 6-well plates (500 cells) and treated with DMSO or lovastatin (4 μM) every other day for 10 days. Formed colonies were stained with crystal violet and counted. Representative images (left) and a summarized graph (right). Error bars, means ± s.d. (n = 3 independent experiments), ∗∗P < 0.01, NS: not significant; Student’s t test (two-tailed). (c) Representative images of PI-staining and flow cytometry using U2OS and KHOS/NP cells treated with MDSO (D) or lovastatin (L) for 72 h (left). Summarized graph showing percentages (%) of sub-G0/G1 fraction and G1/S ratio (right). Error bars, means ± s.d. (n = 3 independent experiments), ∗P < 0.05; Student’s t test (two-tailed). (d) Tumor formation assays in mice subcutaneously injected with CAL33 cells (1 × 106). When subcutaneous tumors reached 3 mm in diameter, mice were intraperitoneally injected with DMSO (D) or atorvastatin (A, 30 mg kg−1) daily, and tumors were measured every 2–3 day until day 21. Representative images of tumors are shown in insets. Error bars, means ± s.d. (n = 5 animals for each group), ∗P < 0.05; Student’s t test (two-tailed). Scale bar, 1 cm. (e) Body weights of mice bearing KHOS/NP-tumors treated with DMSO or atorvastatin (30 mg kg−1, daily). Error bars, means ± s.d. (n = 5 animals for each group). (f) Representative images of IHC for p53, Ki67, and cleaved caspase 3 using CAL33 tumors treated with DMSO or atorvastatin. Scale bar, 50 μm. Statistics source data for a–c are provided in Supplementary Table 1.
Supplementary Figure 5 Response of MEFs with different p53 genotypes to statins.
(a) WB for p53 and vinculin using E1A/H-RasG12V-transformed p53+/+, p53+/R172H, and p53R172H/R172H MEFs treated with DMSO, lovastatin (L, 4 μM), or atorvastatin (A, 4 μM) for 24 h. (b) Quantitative RT-PCR for p21 and PUMA using mRNA from E1A/H-RasG12V-transformed MEFs treated with (+) or without (−) 0.5 μM of doxorubicin for 24 h. Data are shown as relative mRNA expression to that of GAPDH. Error bars, means ± s.d. (n = 3 independent experiments), ∗∗P < 0.01, ∗P < 0.05, NS: not significant; Student’s t test (two-tailed). (c) Colony formation assays using transformed MEFs (500) treated with D or L (4 μM) for 10 days. Representative images (left) and a summarized graph (right). Error bars, means ± s.d. (n = 3 independent experiments), ∗∗P < 0.01; Student’s t test (two-tailed). (d) Representative images of IHC for p53, Ki67, and cleaved caspase 3 using transformed MEFs-derived tumors treated with D or A (magnification, ×40). Scale bar, 50 μm. Statistics source data for b and c are provided in Supplementary Table 1.
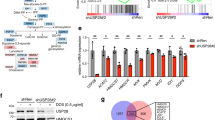
Supplementary Figure 6 Hsp40 (DNAJA1), but not Hsp90, is involved in statin-induced mutp53 degradation, and reduced MVP by statins changes the binding partner of mutp53 from DNAJA1 to CHIP.
(a) WB for Hsp90, Hsp90 client proteins (p53R175H, EGFR, Raf-1, and ErbB2), and β-actin or GAPDH using SK-Br-3 cells treated with DMSO (D) or lovastatin (4 μM). (b) Immunofluorescence for p53R175H using SK-Br-3 cells infected with lentiviral vectors encoding control or DNAJA1 shRNAs and treated with vehicle (ethanol) or leptomycin B (LMB, 50 nM). Scale bar, 50 μm.
Supplementary Figure 7 Reduced MVP inhibits mutp53’s binding to DNAJA1 but increases its interaction with CHIP.
(a) Co-immunoprecipitation and WB for p53R175H and DNAJA1 using CAL33 cells treated with D or L for 11 h, before the degradation of mutp53. (b) Co-immunoprecipitation and WB for p53Y 220C and DNAJA1 using BxPC-3 cells treated with D, L, or L + MVP for 11 h. (c) Co-immunoprecipitation and WB for p53Y 220C and CHIP using BxPC-3 cells treated with D or L for 11 h. (d) A schematic view of a proposed mechanism for statin-induced mutp53 degradation. In the absence of DNAJA1, conformational/misfolded mutp53 is degraded by CHIP, suggesting that DNAJA1 determines the fate of mutp53.
Supplementary information
Supplementary Information
Supplementary Information (PDF 14009 kb)
Supplementary Table 1
Supplementary Information (XLSX 23 kb)
Rights and permissions
About this article
Cite this article
Parrales, A., Ranjan, A., Iyer, S. et al. DNAJA1 controls the fate of misfolded mutant p53 through the mevalonate pathway. Nat Cell Biol 18, 1233–1243 (2016). https://doi.org/10.1038/ncb3427
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3427
This article is cited by
-
Translating p53-based therapies for cancer into the clinic
Nature Reviews Cancer (2024)
-
Precise pancreatic cancer therapy through targeted degradation of mutant p53 protein by cerium oxide nanoparticles
Journal of Nanobiotechnology (2023)
-
Functional significance of cholesterol metabolism in cancer: from threat to treatment
Experimental & Molecular Medicine (2023)
-
Activation of the plant mevalonate pathway by extracellular ATP
Nature Communications (2022)
-
Malic enzyme 2 maintains protein stability of mutant p53 through 2-hydroxyglutarate
Nature Metabolism (2022)