Abstract
Atherosclerosis is primarily a disease of lipid metabolism and inflammation; however, it is also closely associated with endothelial extracellular matrix (ECM) remodelling, with fibronectin accumulating in the laminin–collagen basement membrane. To investigate how fibronectin modulates inflammation in arteries, we replaced the cytoplasmic tail of the fibronectin receptor integrin α5 with that of the collagen/laminin receptor integrin α2. This chimaera suppressed inflammatory signalling in endothelial cells on fibronectin and in knock-in mice. Fibronectin promoted inflammation by suppressing anti-inflammatory cAMP. cAMP was activated through endothelial prostacyclin secretion; however, this was ECM-independent. Instead, cells on fibronectin suppressed cAMP via enhanced phosphodiesterase (PDE) activity, through direct binding of integrin α5 to phosphodiesterase-4D5 (PDE4D5), which induced PP2A-dependent dephosphorylation of PDE4D5 on the inhibitory site Ser651. In vivo knockdown of PDE4D5 inhibited inflammation at athero-prone sites. These data elucidate a molecular mechanism linking ECM remodelling and inflammation, thereby identifying a new class of therapeutic targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Galkina, E. & Ley, K. Immune and inflammatory mechanisms of atherosclerosis. Annu. Rev. Immunol. 27, 165–197 (2009).
Zarins, C. K. et al. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ. Res. 53, 502–514 (1983).
Caro, C. G., Fitz-Gerald, J. M. & Schroter, R. C. Arterial wall shear and distribution of early atheroma in man. Nature 223, 1159–1161 (1969).
Libby, P., Ridker, P. M. & Hansson, G. K. Progress and challenges in translating the biology of atherosclerosis. Nature 473, 317–325 (2011).
Conway, D. E. & Schwartz, M. A. Flow-dependent cellular mechanotransduction in atherosclerosis. J. Cell Sci. 126, 5101–5109 (2013).
Gaudet, A. D. & Popovich, P. G. Extracellular matrix regulation of inflammation in the healthy and injured spinal cord. Exp. Neurol. 258, 24–34 (2014).
Bollyky, P., Bogdani, M., Bollyky, J., Hull, R. & Wight, T. The role of hyaluronan and the extracellular matrix in islet inflammation and immune regulation. Curr. Diab. Rep. 12, 471–480 (2012).
Papageorgiou, A.-P. & Heymans, S. Interactions between the extracellular matrix and inflammation during viral myocarditis. Immunobiology 217, 503–510 (2012).
Grant, D. S., Kleinman, H. K. & Martin, G. R. The role of basement membranes in vascular development. Ann. NY Acad. Sci. 588, 61–72 (1990).
Kim, S., Bell, K., Mousa, S. A. & Varner, J. A. Regulation of angiogenesis in vivo by ligation of integrin α5β1 with the central cell-binding domain of fibronectin. Am. J. Pathol. 156, 1345–1362 (2000).
Chiang, H.-Y., Korshunov, V. A., Serour, A., Shi, F. & Sottile, J. Fibronectin is an important regulator of flow-induced vascular remodeling. Arterioscler. Thromb. 29, 1074–1079 (2009).
Chiu, C.-H., Chou, C.-W., Takada, S. & Liu, Y.-W. Development and fibronectin signaling requirements of the zebrafish interrenal vessel. PLoS ONE 7, e43040 (2012).
Orr, A. W. et al. The subendothelial extracellular matrix modulates NF-κB activation by flow: a potential role in atherosclerosis. J. Cell Biol. 169, 191–202 (2005).
Tan, M. H. et al. Deletion of the alternatively spliced fibronectin EIIIA domain in mice reduces atherosclerosis. Blood 104, 11–18 (2004).
Babaev, V. R. et al. Absence of regulated splicing of fibronectin EDA exon reduces atherosclerosis in mice. Atherosclerosis 197, 534–540.
Rohwedder, I. et al. Plasma fibronectin deficiency impedes atherosclerosis progression and fibrous cap formation. EMBO Mol. Med. 4, 564–576 (2012).
Nagel, T., Resnick, N., Atkinson, W. J., Dewey, C. F. Jr & Gimbrone, M. A. Jr Shear stress selectively upregulates intercellular adhesion molecule-1 expression in cultured human vascular endothelial cells. J. Clin. Invest. 94, 885–891 (1994).
Bao, X., Lu, C. & Frangos, J. A. Temporal gradient in shear but not steady shear stress induces PDGF-A and MCP-1 expression in endothelial cells: role of NO, NFκB, and egr-1. Arterioscler. Thromb. 19, 996–1003 (1999).
Orr, A. W. et al. Matrix-specific p21-activated kinase activation regulates vascular permeability in atherogenesis. J. Cell Biol. 176, 719–727 (2007).
Orr, A. W., Hahn, C., Blackman, B. R. & Schwartz, M. A. p21-activated kinase signaling regulates oxidant-dependent NF-κB activation by flow. Circ. Res. 103, 671–679 (2008).
Hahn, C., Orr, A. W., Sanders, J. M., Jhaveri, K. A. & Schwartz, M. A. The subendothelial extracellular matrix modulates JNK activation by flow. Circ. Res. 104, 995–1003 (2009).
Funk, S. D. et al. Matrix-specific protein kinase a signaling regulates p21-activated kinase activation by flow in endothelial cells. Circ. Res. 106, 1394–1403 (2010).
Yurdagul, A. et al. Altered nitric oxide production mediates matrix-specific PAK2 and NF-κB activation by flow. Mol. Biol. Cell 24, 398–408 (2013).
Yurdagul, A. et al. α5β1 integrin signaling mediates oxidized low-density lipoprotein-induced inflammation and early atherosclerosis. Arterioscler. Thromb. 34, 1362–1373 (2014).
Orr, A. W., Ginsberg, M. H., Shattil, S. J., Deckmyn, H. & Schwartz, M. A. Matrix-specific suppression of integrin activation in shear stress signaling. Mol. Biol. Cell 17, 4686–4697 (2006).
Madamanchi, A., Santoro, S. A. & Zutter, M. M. in I Domain Integrins (ed. Gullberg, D.) 41–60 (Springer, 2014).
Wang, C., Baker, B. M., Chen, C. S. & Schwartz, M. A. Endothelial cell sensing of flow direction. Arterioscler. Thromb. 33, 2130–2136 (2013).
Khachigian, L. M., Resnick, N., Gimbrone, M. A. Jr & Collins, T. Nuclear factor-kappa B interacts functionally with the platelet-derived growth factor B-chain shear-stress response element in vascular endothelial cells exposed to fluid shear stress. J. Clin. Invest. 96, 1169–1175 (1995).
Glagov, S., Zarins, C., Giddens, D. & Ku, D. N. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch. Pathol. Lab. Med. 112, 1018–1031 (1988).
Frangos, S. G., Gahtan, V. & Sumpio, B. Localization of atherosclerosis: role of hemodynamics. Arch. Surg. 134, 1142–1149 (1999).
Frangos, J. A., Eskin, S. G., McIntire, L. V. & Ives, C. L. Flow effects on prostacyclin production by cultured human endothelial cells. Science 227, 1477–1479 (1985).
Fetalvero, K. M., Martin, K. A. & Hwa, J. Cardioprotective prostacyclin signaling in vascular smooth muscle. Prostaglandins Other Lipid Mediat. 82, 109–118 (2007).
Stitham, J., Midgett, C., Martin, K. & Hwa, J. Prostacyclin: an inflammatory paradox. Front. Pharmacol. 2, 24 (2011).
Tsai, M.-C. et al. Shear stress induces synthetic-to-contractile phenotypic modulation in smooth muscle cells via peroxisome proliferator-activated receptor α/δ activations by prostacyclin released by sheared endothelial cells. Circ. Res. 105, 471–480 (2009).
Maurice, D. H. et al. Advances in targeting cyclic nucleotide phosphodiesterases. Nat. Rev. Drug Discov. 13, 290–314 (2014).
Baillie, G. S. Compartmentalized signalling: spatial regulation of cAMP by the action of compartmentalized phosphodiesterases. FEBS J. 276, 1790–1799 (2009).
Muzaffar, S., Jeremy, J. Y., Angelini, G. D. & Shukla, N. NADPH oxidase 4 mediates upregulation of type 4 phosphodiesterases in human endothelial cells. J. Cell. Physiol. 227, 1941–1950 (2012).
Wang, J., Bingaman, S. & Huxley, V. H. Intrinsic sex-specific differences in microvascular endothelial cell phosphodiesterases. Am. J. Physiol. Heart Circ. Physiol. 298, H1146–H1154 (2010).
Netherton, S. J. & Maurice, D. H. Vascular endothelial cell cyclic nucleotide phosphodiesterases and regulated cell migration: implications in angiogenesis. Mol. Pharmacol. 67, 263–272 (2005).
Thompson, W. J. et al. Regulation of cyclic AMP in rat pulmonary microvascular endothelial cells by rolipram-sensitive cyclic AMP phosphodiesterase (PDE4). Biochem. Pharmacol. 63, 797–807 (2002).
McCormick, K. & Baillie, G. S. Compartmentalisation of second messenger signalling pathways. Curr. Opin. Genet. Dev. 27, 20–25 (2014).
Pullamsetti, S. S. et al. cAMP phosphodiesterase inhibitors increases nitric oxide production by modulating dimethylarginine dimethylaminohydrolases. Circulation 123, 1194–1204 (2011).
Hildebrand, J. D., Schaller, M. D. & Parsons, J. T. Identification of sequences required for the efficient localization of the focal adhesion kinase, pp125FAK, to cellular focal adhesions. J. Cell Biol. 123, 993–1005 (1993).
Pfaff, M., Liu, S., Erle, D. J. & Ginsberg, M. H. Integrin β cytoplasmic domains differentially bind to cytoskeletal proteins. J. Biol. Chem. 273, 6104–6109 (1998).
MacKenzie, S. J., Baillie, G. S., McPhee, I., Bolger, G. B. & Houslay, M. D. ERK2 mitogen-activated protein kinase binding, phosphorylation, and regulation of the PDE4D cAMP-specific phosphodiesterases: The involvement of COOH-terminal docking sites and NH2-terminal UCR regions. J. Biol. Chem. 275, 16609–16617 (2000).
Dahlman, J. E. et al. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat. Nanotech. 9, 648–655 (2014).
Plump, A. S. et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 71, 343–353 (1992).
Smith, J. A. Neutrophils, host defense, and inflammation: a double-edged sword. J. Leukoc. Biol. 56, 672–686 (1994).
Morganti-Kossmann, M. C., Rancan, M., Stahel, P. F. & Kossmann, T. Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr. Opin. Crit. Care 8, 101–105 (2002).
Arroyo, A. G. & Iruela-Arispe, M. L. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc. Res. 86, 226–235 (2010).
Sorokin, L. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 10, 712–723 (2010).
Stratman, A. N., Malotte, K. M., Mahan, R. D., Davis, M. J. & Davis, G. E. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 114, 5091–5101 (2009).
Davis, G. E. & Senger, D. R. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ. Res. 97, 1093–1107 (2005).
Hahn, C. & Schwartz, M. A. The role of cellular adaptation to mechanical forces in atherosclerosis. Arterioscler. Thromb. 28, 2101–2107 (2008).
Jongstra-Bilen, J. et al. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J. Exp. Med. 203, 2073–2083 (2006).
Kakolyris, S., Karakitsos, P., Tzardi, M. & Agapitos, E. Immunohistochemical detection of fibronectin in early and advanced atherosclerosis. In Vivo 9, 35–40 (1995).
Ghosh, S. et al. Systems genetics analysis of genome-wide association study reveals novel associations between key biological processes and coronary artery disease. Arterioscler. Thromb. 35, 1712–1722 (2015).
Lee, G.-S. et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492, 123–127 (2012).
Sokolowska, M. et al. Prostaglandin E2 inhibits NLRP3 inflammasome activation through EP4 receptor and intracellular cyclic AMP in human macrophages. J. Immunol. 194, 5472–5487 (2015).
Yan, Y. et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 160, 62–73 (2015).
Libby, P., Okamoto, Y., Rocha, V. Z. & Folco, E. Inflammation in atherosclerosis transition from theory to practice. Circ. J. 74, 213–220 (2010).
Ni, H. et al. Plasma fibronectin promotes thrombus growth and stability in injured arterioles. Proc. Natl Acad. Sci. USA 100, 2415–2419 (2003).
Sakai, T. et al. Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nat. Med. 7, 324–330 (2001).
Allport, J. R. et al. Neutrophils from MMP-9- or neutrophil elastase-deficient mice show no defect in transendothelial migration under flow in vitro. J. Leukoc. Biol. 71, 821–828 (2002).
Tian, J., Alimperti, S., Lei, P. & Andreadis, S. T. Lentiviral microarrays for real-time monitoring of gene expression dynamics. Lab Chip 10, 1967–1975 (2010).
Kim, H. W. et al. Cyclic AMP controls mTOR through regulation of the dynamic interaction between Rheb and phosphodiesterase 4D. Mol. Cell. Biol. 30, 5406–5420 (2010).
Love, K. T. et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc. Natl Acad. Sci. USA 107, 1864–1869 (2010).
Nam, D. et al. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 297, H1535–H1543 (2009).
Paulucci-Holthauzen, A. A. & O’Connor, K. L. Use of pseudosubstrate affinity to measure active protein kinase A. Anal. Biochem. 355, 175–182 (2006).
Yang, T.-T., Sinai, P. & Kain, S. R. An acid phosphatase assay for quantifying the growth of adherent and nonadherent cells. Anal. Biochem. 241, 103–108 (1996).
Acknowledgements
We thank K. Yamada (NIH, USA), D. Calderwood (Yale University, USA), H. Kim (POSTECH, Korea) and A. Jayaraman (Texas A&M University, USA) for providing reagents, and J. Hwa (Yale University, USA) for advice on prostacyclin experiments. Lipid analysis was done by the Yale Mouse Phenotypic Center, supported by a U24 DK059635 grant. This work was funded by a National Institutes of Health grant 5R01HL75092 to M.A.S. G.B. is funded by an MRC project grant (MR/J007412/1). We are grateful to R. Webber and N. Copeland for maintaining the mouse colonies used in this study.
Author information
Authors and Affiliations
Contributions
S.Y. and M.A.S. designed the project. S.Y. performed in vitro experiments and M.B. designed and performed in vivo experiments with the aid of S.Y. J.E.D. prepared and provided nanoparticles. B.G.C. contributed PDE4D5 imaging. R.T.C. performed in vitro PDE assay. R.L. and D.G.A. provided advice on nanoparticle formulation. S.Y. and M.A.S. wrote the manuscript with the contribution of all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
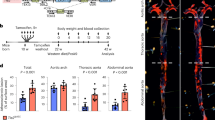
Supplementary Figure 1 Characterization of α5/2 chimera endothelial cells.
(a) BAECs expressing human integrin wild type α5 or the α5/2 chimera were stained with mAb16 that recognizes human integrin α5 extracellular domain. (b) Wild type α5 or the α5/2 chimera cells were lysed and immunoprecipitated with mAb16 to isolate exogenous human integrin α proteins. Western blots with antibodies against the α5 and α2 cytoplasmic tails confirm the sequences (upper and middle panels), and show similar pairing with the β1 subunit (lower panel). (c,d) Wild type α5 and α5/2 chimera cells spreading on fibronectin. BAECs expressing wild type integrin α5 or the chimera were detached and replated on dishes coated with fibronectin (10 μg/ml) for the indicated times. The cells were either fixed and stained with wheat germ agglutinin (c) or lysed and subjected to immunoblotting for FAK phosphorylation (Y397) (d). (e) Wild type α5 or α5/2 chimera cells were plated on fibronectin and grown to monolayer and then kept in low-serum media (1% FBS) for two days to induce fibronectin fibrillogenesis. The cells were fixed and stained for fibronectin. For each condition, n = 30 images pooled across three independent experiments were averaged. The box plot shows the median, with upper and lower percentiles, and the bars show maxima and minima values. (f) Wild type α5 or α5/2 chimera cells were plated on fibronectin and sheared for 36 hrs (20dynes/cm2). Cells were stained with phalloidin and Hoechst and alignment in the direction of flow was quantified (±30°). Scale bars: 50 μm. n = 10 images (60-100 cells/field) were used for quantification for each condition. Error bars are SEM.
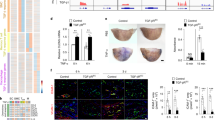
Supplementary Figure 2 PDE4D5 is responsible for ECM-dependent inflammation in endothelial cells.
(a) BAECs on FN or collagen were treated with rolipram (1 μM) and assayed for AMPK activation. (b) Immunoblotting HUVEC lysate with pan PDE4D antibody detects the major band co-migrating with reference PDE4D5 (left panel). The band was diminished by transfection with PDE4D siRNA, demonstrating specificity. Similarly, PDE4D5 was the only isoform detectable in BAEC lysate with immunobotting using pan PDE4D antibody or PDE4D5 specific antibody (right two panels). ∗ indicates non-specific bands. (c) BAECs expressing wild type PDE4D5 or FAT-PDE4D5 were plated on MG for 1hr to monitor GFP signal.
Supplementary Figure 3 PP2A regulates PDE4D5 phosphorylation and inflammation.
(a) Identification of PP2A as a PDE4D5 binding protein. FLAG-PDE4D5 expressing BAECs on FN were lysed and immunoprecipitated with FLAG antibody. Bound protein with size of 35 kDa was submitted for mass analysis and identified as PP2A catalytic subunit. (b) BAECs expressing FLAG-tagged PDE4D5 were plated on either FN or matrigel for indicated times. PDE4D5 was immunoprecipitated with FLAG antibody and eluted with FLAG peptides. Similar results were obtained in 3 experiments. (c) BAECs expressing PDE4D5 wild type were kept in suspension for 90 min then replated on FN-coated dishes for the indicated times. For okadaic acid treatment, cells in suspension were added with 5 nM OA for last 20 min before replating on FN. S651 phosphorylation was assayed by Western blotting (n = 4 independent experiments). (d) BAECs expressing PDE4D5 were plated on FN for 5 hr then pretreated with DMSO or okadaic acid (OA, 5 nM) and then stimulated with IL1β for 30 min. S651 phosphorylation was assayed by Western blotting (n = 3 independent experiments). (e) BAECs expressing PDE4D5 were transfected with a siRNAs targeting PP2A catalytic subunit. The cells were replated on FN then subject to laminar shear for 30 min. (f) BAECs were plated on FN for 5 hr then pretreated with DMSO or okadaic acid (OA, 5 nM) and then stimulated with IL1β for 30 min. NFκB activity was assayed by Western blotting (n = 3 independent experiments). (g) BAECs were transfected with two different siRNAs targeting the PP2A catalytic subunit. The cells were replated on FN then subject to oscillatory shear for 2 hrs (n = 3 independent experiments). Data are represented as means ± SEM. ∗p < 0.05 by one way ANOVA (d,g) or two-tailed t-test (c,f). Source data are provided in Supplementary Table 1.
Supplementary Figure 4 Effect of PP2A inhibition on endothelial cell adhesion.
(a) BAECs were transfected with PP2A siRNA or treated with okadaic acid (OA, 5 nM, 1hr) and plated on FN (10 μg/ml) for 1hr. Cell adhesion was quantified as described in Supplementary Fig. 2 (n = 3 independent experiments) and cell morphology was examined after fixation and phalloidin and nuclear staining. Error bars are SEM. Scale bar: 100 μm. (b) BAECs treated as (a) were plated on FN (10 μg/ml) for indicated times and FAK phosphorylation was measured by Western blotting.
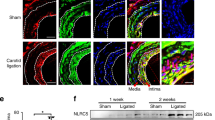
Supplementary Figure 5 In vivo knock-down of endothelial PDE4D.
(a) NIH3T3 cells were transfected with either luciferase siRNA or mouse PDE4D siRNA used for in vivo knock-down. After 72h, PDE4D5 was assayed by Western blotting with tubulin as a loading control. (b) Nano-particles containing PDE4D siRNA or luciferase siRNA (1 mg/kg) were injected intravenously. After two weeks, mouse aortas were isolated, and endothelial expression of PDE4D was assayed by qPCR (n = 4). (c) Serum lipid profile of α5/2; ApoE(-/-) mice after high fat diet (n = 6 mice). Data are represented as means ± SEM. ∗p < 0.05 by two-tailed t-test. Source data are provided in Supplementary Table 1.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1657 kb)
Supplementary Table 1
Supplementary Information (XLS 47 kb)
Rights and permissions
About this article
Cite this article
Yun, S., Budatha, M., Dahlman, J. et al. Interaction between integrin α5 and PDE4D regulates endothelial inflammatory signalling. Nat Cell Biol 18, 1043–1053 (2016). https://doi.org/10.1038/ncb3405
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3405
This article is cited by
-
Pathogenic mechanisms and therapeutic implications of extracellular matrix remodelling in cerebral vasospasm
Fluids and Barriers of the CNS (2023)
-
Effect of cytokine-induced alterations in extracellular matrix composition on diabetic retinopathy-relevant endothelial cell behaviors
Scientific Reports (2022)
-
Phosphodiesterase 4D promotes angiotensin II-induced hypertension in mice via smooth muscle cell contraction
Communications Biology (2022)
-
Associations between SNP83 of phosphodiesterase 4D gene and carotid atherosclerosis in a southern Chinese Han population: a case–control study
Mammalian Genome (2021)
-
Biochemical and mechanical signals in the lymphatic vasculature
Cellular and Molecular Life Sciences (2021)