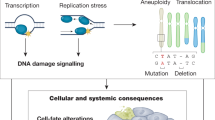
Abstract
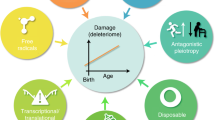
Loss of genome maintenance may causally contribute to ageing, as exemplified by the premature appearance of multiple symptoms of ageing in a growing family of human syndromes and in mice with genetic defects in genome maintenance pathways. Recent evidence revealed a similarity between such prematurely ageing mutants and long-lived mice harbouring mutations in growth signalling pathways. At first sight this seems paradoxical as they represent both extremes of ageing yet show a similar 'survival' response that is capable of delaying age-related pathology and extending lifespan. Understanding the mechanistic basis of this response and its connection with genome maintenance would open exciting possibilities for counteracting cancer or age-related diseases, and for promoting longevity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kirkwood, T. B. Understanding the odd science of aging. Cell 120, 437–447 (2005).
Kirkwood, T. B. & Cremer, T. Cytogerontology since 1881: a reappraisal of August Weismann and a review of modern progress. Hum. Genet. 60, 101–121 (1982).
Guarente, L. & Kenyon, C. Genetic pathways that regulate ageing in model organisms. Nature 408, 255–262 (2000).
Partridge, L. & Gems, D. Mechanisms of ageing: public or private? Nature Rev. Genet. 3, 165–175 (2002).
Kenyon, C. The plasticity of aging: insights from long-lived mutants. Cell 120, 449–460 (2005).
d'Adda di Fagagna, F., Teo, S. H. & Jackson, S. P. Functional links between telomeres and proteins of the DNA-damage response. Genes Dev. 18, 1781–1799 (2004).
Harper, J. W. & Elledge, S. J. The DNA damage response: ten years after. Mol. Cell 28, 739–745 (2007).
Hoeijmakers, J. H. Genome maintenance mechanisms for preventing cancer. Nature 411, 366–374 (2001).
De Bont, R. & van Larebeke, N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis 19, 169–185 (2004).
Sander, M. et al. Proceedings of a workshop on DNA adducts: biological significance and applications to risk assessment Washington, DC, April 13–14, 2004. Toxicol. Appl. Pharmacol. 208, 1–20 (2005).
Lindahl, T. Instability and decay of the primary structure of DNA. Nature 362, 709–715 (1993).
Grillari, J., Katinger, H. & Voglauer, R. Contributions of DNA interstrand cross-links to aging of cells and organisms. Nucleic Acids Res. 35, 7566–7576 (2007).
Beeharry, N. & Broccoli, D. Telomere dynamics in response to chemotherapy. Curr. Mol. Med. 5, 187–196 (2005).
Vijg, J. Somatic mutations and aging: a re-evaluation. Mutat. Res. 447, 117–135 (2000).
Friedberg EC, W. G., Siede W., Wood R. D., Schultz R. A. & Ellenberger T. DNA Repair and Mutagenesis 2nd edn, (ASM, Washington DC, 2006).
Plosky, B. S. & Woodgate, R. Switching from high-fidelity replicases to low-fidelity lesion-bypass polymerases. Curr. Opin. Genet. Dev. 14, 113–119 (2004).
Martin, G. M. Genetic modulation of senescent phenotypes in Homo sapiens. Cell 120, 523–532 (2005).
Vilhem Bohr, D. W., de Souza Pinto, N. C., van der Pluijm, I. & Hoeijmakers, J. H. DNA Repair and Aging, (Cold Spring Harbor Laboratory Press, New York, 2008).
Hasty, P. & Vijg, J. Rebuttal to Miller: 'Accelerated aging': a primrose path to insight?' Aging Cell 3, 67–69 (2004).
Miller, R. A. 'Accelerated aging': a primrose path to insight? Aging Cell 3, 47–51 (2004).
Partridge, L. & Gems, D. Benchmarks for ageing studies. Nature 450, 165–167 (2007).
Campisi, J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522 (2005).
Wijnhoven, S. W. et al. Accelerated aging pathology in ad libitum fed Xpd(TTD) mice is accompanied by features suggestive of caloric restriction. DNA Repair 4, 1314–1324 (2005).
Niedernhofer, L. J. et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature 444, 1038–1043 (2006).
de Boer, J. et al. Premature aging in mice deficient in DNA repair and transcription. Science 296, 1276–1279 (2002).
Hasty, P., Campisi, J., Hoeijmakers, J., van Steeg, H. & Vijg, J. Aging and genome maintenance: lessons from the mouse? Science 299, 1355–1359 (2003).
Scharer, O. D. A molecular basis for damage recognition in eukaryotic nucleotide excision repair. Chembiochem. 9, 21–23 (2008).
Bootsma, D. K., K. H., Cleaver, J. E. & Hoeijmakers, J. H. J. The Metabolic and Molecular Basis of inherited Disease, 677–703 (McGraw-Hill, New York, 2001).
Dolle, M. E. et al. Increased genomic instability is not a prerequisite for shortened lifespan in DNA repair deficient mice. Mutat. Res. 596, 22–35 (2006).
van der Pluijm, I. et al. Impaired genome maintenance suppresses the growth hormone–insulin-like growth factor 1 axis in mice with Cockayne syndrome. PLoS Biol. 5, e2 (2006).
de Boer, J. et al. Mouse model for the DNA repair/basal transcription disorder trichothiodystrophy reveals cancer predisposition. Cancer Res. 59, 3489–3494 (1999).
Hanawalt, P. C. Subpathways of nucleotide excision repair and their regulation. Oncogene 21, 8949–8956 (2002).
Ljungman, M. & Lane, D. P. Transcription — guarding the genome by sensing DNA damage. Nature Rev. Cancer 4, 727–737 (2004).
Andressoo, J. O., Hoeijmakers, J. H. & Mitchell, J. R. Nucleotide excision repair disorders and the balance between cancer and aging. Cell Cycle 5, 2886–2888 (2006).
Li, H., Vogel, H., Holcomb, V. B., Gu, Y. & Hasty, P. Deletion of Ku70, Ku80, or both causes early aging without substantially increased cancer. Mol. Cell Biol. 27, 8205–8214 (2007).
Vogel, H., Lim, D. S., Karsenty, G., Finegold, M. & Hasty, P. Deletion of Ku86 causes early onset of senescence in mice. Proc. Natl Acad. Sci. USA 96, 10770–10775 (1999).
Andressoo, J. O. et al. An Xpd mouse model for the combined xeroderma pigmentosum/Cockayne syndrome exhibiting both cancer predisposition and segmental progeria. Cancer Cell 10, 121–132 (2006).
van de Ven, M. et al. Adaptive stress response in segmental progeria resembles long-lived dwarfism and calorie restriction in mice. PLoS Genet. 2, e192 (2006).
Schumacher, B., Garinis, G. A. & Hoeijmakers, J. H. Age to survive: DNA damage and aging. Trends Genet. 24, 77–85 (2008).
Schumacher, B. et al. Delayed and accelerated aging share common longevity assurance mechanisms. PLoS Genet. 4, e1000161 (2008).
Bartke, A. Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology 146, 3718–3723 (2005).
Li, S. et al. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature 347, 528–533 (1990).
Andersen, B. et al. The Ames dwarf gene is required for Pit-1 gene activation. Dev. Biol. 172, 495–503 (1995).
Godfrey, P. et al. GHRH receptor of little mice contains a missense mutation in the extracellular domain that disrupts receptor function. Nature Genet. 4, 227–232 (1993).
Flurkey, K., Papaconstantinou, J., Miller, R. A. & Harrison, D. E. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl Acad. Sci. USA 98, 6736–6741 (2001).
Zhou, Y. et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc. Natl Acad. Sci. USA 94, 13215–13220 (1997).
Coschigano, K. T., Clemmons, D., Bellush, L. L. & Kopchick, J. J. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology 141, 2608–2613 (2000).
Holzenberger, M. et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 421, 182–187 (2003).
Liu, J. P., Baker, J., Perkins, A. S., Robertson, E. J. & Efstratiadis, A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75, 59–72 (1993).
Kurosu, H. et al. Suppression of aging in mice by the hormone Klotho. Science 309, 1829–1833 (2005).
McCay, C. M., Crowell, M. F. & Maynard, L. A. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition 5, 155–171 (1989).
Maeda, H. et al. Nutritional influences on aging of Fischer 344 rats: II. Pathology. J. Gerontol. 40, 671–688 (1985).
Masoro, E. J. Dietary restriction and aging. J. Am. Geriatr. Soc. 41, 994–999 (1993).
Masoro, E. J. Food restriction in rodents: an evaluation of its role in the study of aging. J. Gerontol. 43, B59–B64 (1988).
Miller, R. A. et al. Gene expression patterns in calorically restricted mice: partial overlap with long-lived mutant mice. Mol. Endocrinol. 16, 2657–2666 (2002).
Bauer, M. et al. Starvation response in mouse liver shows strong correlation with life-span-prolonging processes. Physiol. Genomics 17, 230–244 (2004).
Tsuchiya, T. et al. Additive regulation of hepatic gene expression by dwarfism and caloric restriction. Physiol. Genomics 17, 307–315 (2004).
Garcia, A. M. et al. Effect of Ames dwarfism and caloric restriction on spontaneous DNA mutation frequency in different mouse tissues. Mech. Ageing Dev. 129, 528–533 (2008).
Liang, H. et al. Genetic mouse models of extended lifespan. Exp. Gerontol. 38, 1353–1364 (2003).
Bartke, A. et al. Effects of growth hormone overexpression and growth hormone resistance on neuroendocrine and reproductive functions in transgenic and knock-out mice. Proc. Soc. Exp. Biol. Med. 222, 113–123 (1999).
Cao, S. X., Dhahbi, J. M., Mote, P. L. & Spindler, S. R. Genomic profiling of short- and long-term caloric restriction effects in the liver of aging mice. Proc. Natl Acad. Sci. USA 98, 10630–10635 (2001).
Harman, D. The aging process. Proc. Natl Acad. Sci. USA 78, 7124–7128 (1981).
der Pluijm I, G. G. et al. Impaired genome maintenance suppresses the growth hormone–insulin-like growth factor 1 axis in mice with cockayne syndrome. PLoS Biol. 5, 23–38 (2006).
Yang, H., Baur, J. A., Chen, A., Miller, C. & Sinclair, D. A. Design and synthesis of compounds that extend yeast replicative lifespan. Aging Cell 6, 35–43 (2007).
Acknowledgements
This work was supported by the Netherlands Organization for Scientific Research (NWO) through the foundation of the Research Institute Diseases of the Elderly, as well as grants from SenterNovem IOP-Genomics (IGE03009), NIH (1PO1 AG17242-02), NIEHS (1UO1 ES011044), EC (QRTL-1999-02002), and the Dutch Cancer Society (EUR 99-2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garinis, G., van der Horst, G., Vijg, J. et al. DNA damage and ageing: new-age ideas for an age-old problem. Nat Cell Biol 10, 1241–1247 (2008). https://doi.org/10.1038/ncb1108-1241
Issue Date:
DOI: https://doi.org/10.1038/ncb1108-1241
This article is cited by
-
ROS production by mitochondria: function or dysfunction?
Oncogene (2024)
-
Telomeres and Mitochondrial Metabolism: Implications for Cellular Senescence and Age-related Diseases
Stem Cell Reviews and Reports (2022)
-
The splicing factor XAB2 interacts with ERCC1-XPF and XPG for R-loop processing
Nature Communications (2021)
-
Beyond DNA repair and chromosome instability—Fanconi anaemia as a cellular senescence-associated syndrome
Cell Death & Differentiation (2021)
-
A stress-induced miR-31–CLOCK–ERK pathway is a key driver and therapeutic target for skin aging
Nature Aging (2021)