Abstract
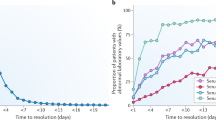
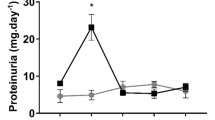
The Predictive Safety Testing Consortium's first regulatory submission to qualify kidney safety biomarkers revealed two deficiencies. To address the need for biomarkers that monitor recovery from agent-induced renal damage, we scored changes in the levels of urinary biomarkers in rats during recovery from renal injury induced by exposure to carbapenem A or gentamicin. All biomarkers responded to histologic tubular toxicities to varied degrees and with different kinetics. After a recovery period, all biomarkers returned to levels approaching those observed in uninjured animals. We next addressed the need for a serum biomarker that reflects general kidney function regardless of the exact site of renal injury. Our assay for serum cystatin C is more sensitive and specific than serum creatinine (SCr) or blood urea nitrogen (BUN) in monitoring generalized renal function after exposure of rats to eight nephrotoxicants and two hepatotoxicants. This sensitive serum biomarker will enable testing of renal function in animal studies that do not involve urine collection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bonventre, J.V. et al. Next-generation biomarkers for detecting kidney toxicity. Nat. Biotechnol. 28, 436–440 (2010).
Ferguson, M.A., Vaidya, V.S. & Bonventre, J.V. Biomarkers of nephrotoxic acute kidney injury. Toxicology 245, 182–193 (2008).
Vaidya, V.S., Ramirez, V., Ichimura, T., Bobadilla, N.A. & Bonventre, J.V. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am. J. Physiol. Renal Physiol. 290, F517–F529 (2006).
Han, W.K. et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 73, 863–869 (2008).
Yu, Y., Jin, H., Holder, D., Ozer, J.S. & Villarreal, S. Urinary biomarkers trefoil factor 3 and albumin enable early detection of kidney tubular injury. Nat. Biotechnol. 28, 470–477 (2010).
Dieterle, F. et al. Urinary clusterin, cystatin C, β2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat. Biotechnol. 28, 463–469 (2010).
Vaidya, V.S. et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat. Biotechnol. 28, 478–485 (2010).
Mattes, W.B. & Walker, E.G. Translational toxicology and the work of the predictive safety testing consortium. Clin. Pharmacol. Ther. 85, 327–330 (2009).
Razzaque, M.S. & Taguchi, T. Cellular and molecular events leading to renal tubulointerstitial fibrosis. Med. Electron Microsc. 35, 68–80 (2002).
Westhuyzen, J. et al. Measurement of tubular enzymuria facilitates early detection of acute renal impairment in the intensive care unit. Nephrol. Dial. Transplant. 18, 543–551 (2003).
Bailly, V. et al. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J. Biol. Chem. 277, 39739–39748 (2002).
Ichimura, T., Hung, C.C., Yang, S.A., Stevens, J.L. & Bonventre, J.V. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am. J. Physiol. Renal Physiol. 286, F552–F563 (2004).
Berger, T. et al. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 103, 1834–1839 (2006).
Silkensen, J.R., Agarwal, A., Nath, K.A., Manivel, J.C. & Rosenberg, M.E. Temporal induction of clusterin in cisplatin nephrotoxicity. J. Am. Soc. Nephrol. 8, 302–305 (1997).
Orlandi, A. et al. Modulation of clusterin isoforms is associated with all-trans retinoic acid-induced proliferative arrest and apoptosis of intimal smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 25, 348–353 (2005).
Vaidya, V.S. & Bonventre, J.V. Mechanistic biomarkers for cytotoxic acute kidney injury. Expert Opin. Drug Metab. Toxicol. 2, 697–713 (2006).
Hoffmann, W. Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell. Mol. Life Sci. 62, 2932–2938 (2005).
Mussap, M. & Plebani, M. Biochemistry and clinical role of human cystatin C. Crit. Rev. Clin. Lab. Sci. 41, 467–550 (2004).
Takuwa, S., Ito, Y., Ushijima, K. & Uchida, K. Serum cystatin-C values in children by age and their fluctuation during dehydration. Pediatr. Int. 44, 28–31 (2002).
Madero, M., Sarnak, M.J. & Stevens, L.A. Serum cystatin C as a marker of glomerular filtration rate. Curr. Opin. Nephrol. Hypertens. 15, 610–616 (2006).
Dharnidharka, V.R., Kwond, C. & Stevens, G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am. J. Kidney Dis. 40, 221–226 (2002).
Shlipak, M.G., Praught, M.L. & Sarnak, M.J. Update on cystatin C: new insights into the importance of mild kidney dysfunction. Curr. Opin. Nephrol. Hypertens. 15, 270–275 (2006).
Herget-Rosenthal, S. et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 66, 1115–1122 (2004).
Anonymous US Food and Drug Administration Agency 510(k) Substantial equivalence determination decision summary device only. (FDA, Rockville, Maryland, USA, 2007) <http://www.fda.gov/cdrh/reviews/K041878.pdf>.
Sing, T., Sander, O., Beerenwinkel, N. & Lengauer, T. ROCR: visualizing classifier performance in R. Bioinformatics 21, 3940–3941 (2005).
Hanley, J.A. & McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143, 29–36 (1982).
DeLong, E.R., DeLong, D.M. & Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44, 837–845 (1988).
Rosen, H. et al. Reduced immunotoxicity and preservation of antibacterial activity in a releasable side-chain carbapenem antibiotic. Science 283, 703–706 (1999).
Sistare, F.D. et al. Towards consensus practices to qualify safety biomarkers for use in early drug development. Nat. Biotechnol. 28, 446–454 (2010).
Acknowledgements
S. Leuillet and B. Palate (Centre International de Toxicologie (CIT)) kindly performed Novartis studies and the histopathology assessment and J. Mapes (RBM) developed the S-cystatin C assay. We thank G. Miller and P. Srinivasa for helpful comments on the manuscript. Z.E., K.V. and W.E.G. kindly shared unpublished observations for GST alpha.
Author information
Authors and Affiliations
Contributions
J.S.O., F.D., W.J.B., M.J.T., T.R.S., J.F.S., W.E.G., E.P., A.C., F.S., A.M., O.G., D.R.R., F.L., S.-D.C., G.M., J.V., D.L.G., F.D.S. and D.W. designed research; Z.E., T.F., N.M., E.P., D.R.R., S.T., H.K.C., S.R., D.T.T., K.V. and H.J. performed research; Z.E. and K.V. contributed new reagents/analytic tools; J.S.O., D.H., N.M., W.E.G., F.D., Y.Y., G.M., P.V., A.C., D.L.G. and F.D.S. analyzed data; and J.S.O., S.T., Z.E., K.V., F.D., D.L.G. and F.D.S. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
All authors are present or past employees of Merck or Novartis.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1,2 and Supplementary Figs. 1–4 (PDF 655 kb)
Rights and permissions
About this article
Cite this article
Ozer, J., Dieterle, F., Troth, S. et al. A panel of urinary biomarkers to monitor reversibility of renal injury and a serum marker with improved potential to assess renal function. Nat Biotechnol 28, 486–494 (2010). https://doi.org/10.1038/nbt.1627
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.1627
This article is cited by
-
The evolving role of investigative toxicology in the pharmaceutical industry
Nature Reviews Drug Discovery (2023)
-
Renoprotective effects of estrogen on acute kidney injury: the role of SIRT1
International Urology and Nephrology (2021)
-
CXCL8(3–72) K11R/G31P protects against sepsis-induced acute kidney injury via NF-κB and JAK2/STAT3 pathway
Biological Research (2019)
-
Molecular optical imaging probes for early diagnosis of drug-induced acute kidney injury
Nature Materials (2019)
-
Kidney-based in vitro models for drug-induced toxicity testing
Archives of Toxicology (2019)