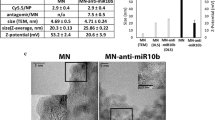
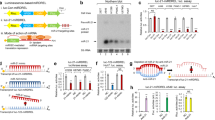
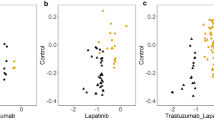
Abstract
MicroRNAs (miRNAs) are increasingly implicated in the regulation of metastasis. Despite their potential as targets for anti-metastatic therapy, miRNAs have only been silenced in normal tissues of rodents and nonhuman primates. Therefore, the development of effective approaches for sequence-specific inhibition of miRNAs in tumors remains a scientific and clinical challenge. Here we show that systemic treatment of tumor-bearing mice with miR-10b antagomirs—a class of chemically modified anti-miRNA oligonucleotide—suppresses breast cancer metastasis. Both in vitro and in vivo, silencing of miR-10b with antagomirs significantly decreases miR-10b levels and increases the levels of a functionally important miR-10b target, Hoxd10. Administration of miR-10b antagomirs to mice bearing highly metastatic cells does not reduce primary mammary tumor growth but markedly suppresses formation of lung metastases in a sequence-specific manner. The miR-10b antagomir, which is well tolerated by normal animals, appears to be a promising candidate for the development of new anti-metastasis agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fidler, I.J. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat. Rev. Cancer 3, 453–458 (2003).
Steeg, P.S. Tumor metastasis: mechanistic insights and clinical challenges. Nat. Med. 12, 895–904 (2006).
Ma, L. & Weinberg, R.A. Micromanagers of malignancy: role of microRNAs in regulating metastasis. Trends Genet. 24, 448–456 (2008).
Nicoloso, M.S., Spizzo, R., Shimizu, M., Rossi, S. & Calin, G.A. MicroRNAs—the micro steering wheel of tumour metastases. Nat. Rev. Cancer 9, 293–302 (2009).
Steeg, P.S. & Theodorescu, D. Metastasis: a therapeutic target for cancer. Nat. Clin. Pract. Oncol. 5, 206–219 (2008).
Romond, E.H. et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 353, 1673–1684 (2005).
Piccart-Gebhart, M.J. et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 353, 1659–1672 (2005).
Yang, J.C. et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N. Engl. J. Med. 349, 427–434 (2003).
Hurwitz, H. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350, 2335–2342 (2004).
Bartel, D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004).
He, L. & Hannon, G.J. MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. 5, 522–531 (2004).
Ma, L., Teruya-Feldstein, J. & Weinberg, R.A. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 449, 682–688 (2007).
Huang, Q. et al. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat. Cell Biol. 10, 202–210 (2008).
Tavazoie, S.F. et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature 451, 147–152 (2008).
Asangani, I.A. et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 27, 2128–2136 (2008).
Zhu, S. et al. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 18, 350–359 (2008).
Valastyan, S. et al. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell 137, 1032–1046 (2009).
Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2, 442–454 (2002).
Yang, J. et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117, 927–939 (2004).
Edmonds, M.D. et al. Breast cancer metastasis suppressor 1 coordinately regulates metastasis-associated microRNA expression. Int. J. Cancer 125, 1778–1785 (2009).
Gee, H.E. et al. MicroRNA-10b and breast cancer metastasis. Nature 455, E8–E9; author reply, 455, E9 (2008).
Baffa, R. et al. MicroRNA expression profiling of human metastatic cancers identifies cancer gene targets. J. Pathol. 219, 214–221 (2009).
Bloomston, M. et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. J. Am. Med. Assoc. 297, 1901–1908 (2007).
Ciafre, S.A. et al. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem. Biophys. Res. Commun. 334, 1351–1358 (2005).
Hao-Xiang, T. et al. MicroRNA-9 reduces cell invasion and E-cadherin secretion in SK-Hep-1 cell. Med. Oncol. published online, doi:10.1007/s12032-009-9264-2 (2 July 2009).
Sasayama, T., Nishihara, M., Kondoh, T., Hosoda, K. & Kohmura, E. MicroRNA-10b is overexpressed in malignant glioma and associated with tumor invasive factors, uPAR and RhoC. Int. J. Cancer 125, 1407–1413 (2009).
Krutzfeldt, J. et al. Specificity, duplex degradation and subcellular localization of antagomirs. Nucleic Acids Res. 35, 2885–2892 (2007).
Krutzfeldt, J. et al. Silencing of microRNAs in vivo with 'antagomirs'. Nature 438, 685–689 (2005).
Esau, C. et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 3, 87–98 (2006).
Elmen, J. et al. LNA-mediated microRNA silencing in non-human primates. Nature 452, 896–899 (2008).
Aslakson, C.J. & Miller, F.R. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 52, 1399–1405 (1992).
Davis, S. et al. Potent inhibition of microRNA in vivo without degradation. Nucleic Acids Res. 37, 70–77 (2009).
Li, Q.J. et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell 129, 147–161 (2007).
Wurdinger, T. et al. miR-296 regulates growth factor receptor overexpression in angiogenic endothelial cells. Cancer Cell 14, 382–393 (2008).
Yi, R., Poy, M.N., Stoffel, M. & Fuchs, E. A skin microRNA promotes differentiation by repressing 'stemness'. Nature 452, 225–229 (2008).
Iorio, M.V. et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 65, 7065–7070 (2005).
Ebert, M.S., Neilson, J.R. & Sharp, P.A. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat. Methods 4, 721–726 (2007).
Weiss, F.U. et al. Retinoic acid receptor antagonists inhibit miR-10a expression and block metastatic behavior of pancreatic cancer. Gastroenterology 137, 2136–2145 (2009).
Dykxhoorn, D.M. et al. miR-200 enhances mouse breast cancer cell colonization to form distant metastases. PLoS ONE 4, e7181 (2009).
Bernards, R. & Weinberg, R.A. A progression puzzle. Nature 418, 823 (2002).
Talmadge, J.E. Clonal selection of metastasis within the life history of a tumor. Cancer Res. 67, 11471–11475 (2007).
Scheel, C., Onder, T., Karnoub, A. & Weinberg, R.A. Adaptation versus selection: the origins of metastatic behavior. Cancer Res. 67, 11476–11479; discussion, 67, 11479–11480 (2007).
Krichevsky, A.M. & Gabriely, G. miR-21: a small multi-faceted RNA. J. Cell. Mol. Med. 13, 39–53 (2009).
Wang, P. et al. microRNA-21 negatively regulates Cdc25A and cell cycle progression in colon cancer cells. Cancer Res. 69, 8157–8165 (2009).
Park, J.K., Lee, E.J., Esau, C. & Schmittgen, T.D. Antisense inhibition of microRNA-21 or -221 arrests cell cycle, induces apoptosis, and sensitizes the effects of gemcitabine in pancreatic adenocarcinoma. Pancreas 38, e190–e199 (2009).
Huse, J.T. & Holland, E.C. Yin and yang: cancer-implicated miRNAs that have it both ways. Cell Cycle 8, 3611–3612 (2009).
Acknowledgements
We thank C.L. Daige at Regulus for technical assistance, M. Ebert, P. Sharp and S. Valastyan for advice on miRNA sponge design, the Histology Core Labs at Massachusetts Institute of Technology (MIT) and Memorial Sloan-Kettering Cancer Center for assistance with histology, B. Bierie and other members of the Weinberg Lab for useful discussions. L.M. is a recipient of a Life Sciences Research Foundation Fellowship, a Margaret and Herman Sokol Award and a National Institutes of Health (NIH) Pathway to Independence Award (K99/R00). R.A.W. is an American Cancer Society Research Professor and a D.K. Ludwig Cancer Research Professor. This research is supported by an NIH grant to R.A.W. and the Ludwig Center for Molecular Oncology at MIT.
Author information
Authors and Affiliations
Contributions
R.A.W. supervised research. L.M., J.S. and E.G.M. designed experiments. J.S., B.B. and E.G.M. provided antagomirs and conditions. L.M., F.R. and E.P. performed most of the experiments. E.G.M. led toxicity assessment at Regulus. J.T.-F. performed pathological analysis. G.W.B. contributed to graphics and statistical analysis. L.M. and R.A.W. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.S., B.B. and E.M. are employees of Regulus Therapeutics.
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–6 (PDF 13954 kb)
Rights and permissions
About this article
Cite this article
Ma, L., Reinhardt, F., Pan, E. et al. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol 28, 341–347 (2010). https://doi.org/10.1038/nbt.1618
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.1618
This article is cited by
-
MicroRNA: trends in clinical trials of cancer diagnosis and therapy strategies
Experimental & Molecular Medicine (2023)
-
Novel sights on therapeutic, prognostic, and diagnostics aspects of non-coding RNAs in glioblastoma multiforme
Metabolic Brain Disease (2023)
-
Diagnostic applications and therapeutic option of Cascade CRISPR/Cas in the modulation of miRNA in diverse cancers: promises and obstacles
Journal of Cancer Research and Clinical Oncology (2023)
-
A perspective on the metastasis suppressor field
Cancer and Metastasis Reviews (2023)
-
Comprehensive microRNA analysis across genome-edited colorectal cancer organoid models reveals miR-24 as a candidate regulator of cell survival
BMC Genomics (2022)