Abstract
Two distinct forms of natural selection promote adaptive biological diversity. Divergent selection occurs when different environments favour different phenotypes, leading to increased differences between populations1. Negative frequency-dependent selection occurs when rare variants within a population are favoured over common ones2, increasing diversity within populations3. These two diversifying forces promote genetic variation at different spatial scales, and may act in opposition, but their relative effects remain unclear because they are rarely measured concurrently. Here we show that negative frequency-dependent selection within populations can favor rare immigrants over locally adapted residents. We reciprocally transplanted lake and stream ecotypes of threespine stickleback4 into lake and stream habitats, while manipulating the relative abundance of residents versus immigrants. We found negative frequency-dependence: survival was highest for the locally rare ecotype, rather than natives. Also, individuals with locally rare major histocompatibility complex (MHC) class IIb genotypes were infected by fewer parasites. This negative frequency-dependent selection will tend to favour rare immigrants over common residents, amplifying the effect of migration and undermining the efficacy of divergent natural selection to drive population differences. The only signal of divergent selection was a tendency for foreign fish to have higher parasite loads than residents, after controlling for MHC genotype rarity. Frequency-dependent ecological interactions have long been thought to promote speciation. Our results suggest a more nuanced view in which negative frequency dependence alters the fate of migrants to promote or constrain evolutionary divergence between populations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fisher, R. A. The Genetical Theory of Natural Selection (Clarendon, 1930)
Ayala, F. J. & Campbell, C. A. Frequency-dependent selection. Annu. Rev. Ecol. Syst. 5, 115–138 (1974)
Kopp, M. & Hermisson, J. The evolution of genetic architecture under frequency-dependent disruptive selection. Evolution 60, 1537–1550 (2006)
Hendry, A. P. & Taylor, E. B. How much of the variation in adaptive divergence can be explained by gene flow? An evaluation using lake-stream stickleback pairs. Evolution 58, 2319–2331 (2004)
Shafer, A. B. A. & Wolf, J. B. W. Widespread evidence for incipient ecological speciation: a meta-analysis of isolation-by-ecology. Ecol. Lett. 16, 940–950 (2013)
Savolainen, O., Lascoux, M. & Merilä, J. Ecological genomics of local adaptation. Nat. Rev. Genet. 14, 807–820 (2013)
Kawecki, T. J. & Ebert, D. Conceptual issues in local adaptation. Ecol. Lett. 7, 1225–1241 (2004)
Rundle, H. D. & Nosil, P. Ecological speciation. Ecol. Lett. 8, 336–352 (2005)
Lenormand, T. Gene flow and the limits to natural selection. Trends Ecol. Evol. 17, 183–189 (2002)
Doebeli, M. & Dieckmann, U. Evolutionary branching and sympatric speciation caused by different types of ecological interactions. Am. Nat. 156, S77–S101 (2000)
Bolnick, D. I. & Fitzpatrick, B. M. Sympatric speciation: models and empirical evidence. Annu. Rev. Ecol. Evol. Syst. 38, 459–487 (2007)
Gray, S. M. & McKinnon, J. S. Linking color polymorphism maintenance and speciation. Trends Ecol. Evol. 22, 71–79 (2007)
Ebert, D. et al. A selective advantage to immigrant genes in a Daphnia metapopulation. Science 295, 485–488 (2002)
Hereford, J. A quantitative survey of local adaptation and fitness trade-offs. Am. Nat. 173, 579–588 (2009)
Schluter, D. Frequency dependent natural selection during character displacement in sticklebacks. Evolution 57, 1142–1150 (2003)
Antonovics, J. & Kareiva, P. Frequency-dependent selection and competition: empirical approaches. Phil. Trans. R. Soc. Ser. B 319, 601–613 (1988)
Hendry, A. P., Bolnick, D. I., Berner, D. & Peichel, C. L. Along the speciation continuum in sticklebacks. J. Fish Biol. 75, 2000–2036 (2009)
Bolnick, D. I. Can intraspecific competition drive disruptive selection? An experimental test in natural populations of sticklebacks. Evolution 58, 608–618 (2004)
Wegner, K. M., Kalbe, M., Milinski, M. & Reusch, T. B. Mortality selection during the 2003 European heat wave in three-spined sticklebacks: effects of parasites and MHC genotype. BMC Evol. Biol. 8, 124 (2008)
Vasseur, D. A., Amarasekare, P., Rudolf, V. H. W. & Levine, J. M. Eco-evolutionary dynamics enable coexistence via neighbor-dependent selection. Am. Nat. 178, E96–E109 (2011)
Svanbäck, R. & Bolnick, D. I. Intraspecific competition drives increased resource use diversity within a natural population. Proc. R. Soc. B 274, 839–844 (2007)
Ingram, T., Stutz, W. E. & Bolnick, D. I. Does intraspecific size variation in a predator affect its diet diversity and top-down control of prey? PLoS One 6, e20782 (2011)
Rauch, G., Kalbe, M. & Reusch, T. B. H. Relative importance of MHC and genetic background for parasite load in a field experiment. Evol. Ecol. Res. 8, 373–386 (2006)
Spurgin, L. G. & Richardson, D. S. How pathogens drive genetic diversity: MHC, mechanisms and misunderstandings. Proc. R. Soc. B 277, 979–988 (2010)
Eizaguirre, C., Lenz, T. L., Kalbe, M. & Milinski, M. Divergent selection on locally adapted Major Histocompatibility Complex immune genes experimentally proven in the field. Ecol. Lett. 15, 723–731 (2012)
Bolnick, D. I., Hendrix, K. A., Jordan, L. A., Veen, T. & Brock, C. D. Intruder color and light environment jointly determine how nesting male stickleback respond to simulated territorial intrusions. Biol. Lett. 12, 20160467 (2016)
Hughes, R. N. & Croy, M. I. An experimental analysis of frequency-dependent predation (switching) in the 15-spined stickleback, Spinachia spinachia. J. Anim. Ecol. 62, 341–352 (1993)
Nosil, P. Reproductive isolation caused by visual predation on migrants between divergent environments. Proc. R. Soc. B 271, 1521–1528 (2004)
Gurevitch, J., Morrow, L. L., Wallace, A. & Walsh, J. S. A meta-analysis of competition in field experiments. Am. Nat. 140, 539–572 (1992)
Fumagalli, M. et al. Signatures of environmental genetic adaptation pinpoint pathogens as the main selective pressure through human evolution. PLoS Genet. 7, e1002355 (2011)
Weber, J., Bradburd, G. S., Stuart, Y. E., Stutz, W. E. & Bolnick, D. I. The relative contributions of distance, landscape resistance, and habitat, to genomic divergence between parapatric lake and stream stickleback. Evolution 71, 342–356 (2017)
Stutz, W. E. & Bolnick, D. I. A Stepwise Threshold Clustering (STC) method to infer genotypes from error-prone next-generation sequencing of multi-allele genes such as the Major Histocompatibility Complex (MHC). PLoS One 9, e100587 (2014)
Oke, K. et al. Plasticity enhances phenotypic parallelism: evidence from lake-stream stickleback. J. Evol. Biol. 29, 126–143 (2016)
Berner, D., Grandchamp, A.-C. & Hendry, A. P. Variable progress toward ecological speciation in parapatry: stickleback across eight lake-stream transitions. Evolution 63, 1740–1753 (2009)
Bolnick, D. I. et al. Ecological release from interspecific competition leads to decoupled changes in population and individual niche width. Proc. R. Soc. B 277, 1789–1797 (2010)
Stutz, W. E., Coates, J., Schmerer, M. & Bolnick, D. Among-population divergence in stickleback immune gene expression is predominantly environmentally-induced, rather than heritable. Mol. Ecol. 24, 4629–4646 (2015)
R Development Core Team R: a Language and Environment for Statistical Computing. http://www.R-project.org (R Foundation for Statistical Computing, 2008)
Bolnick, D. & Otto, S. The magnitude of local adaptation under genotype-dependent dispersal. Ecol. Evol. 3, 4733–4735 (2013)
Acknowledgements
We thank L. Snowberg, K. Ballare, G. Calabrese, J. Heiling, K. Jiang, C. Brock and M. Bright for field assistance. This project was supported by grants to D.I.B. from the Howard Hughes Medical Institute and the NSF (DEB-1144773 and DEB-1456462) and grants to W.E.S. from the EEB Graduate Program at the University of Texas.
Author information
Authors and Affiliations
Contributions
The experiment was jointly designed by W.E.S. and D.I.B. The field research, sequencing, and specimen measurements were conducted by W.E.S. Both authors contributed to data analysis. The manuscript was written by D.I.B. with input from W.E.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks R. Calsbeek, J. McKinnon and D. Reznick for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Simulations of how migration, NFDS and DS jointly affect the equilibrium evolutionary divergence between populations.
a, To illustrate the effect of NFDS on between-population divergence, here we plot a single instance of a simulation in which we began with two non-diverged populations (allele frequencies pA = pB = 0.5). Simulation details are provided below. In a we plot the process of population divergence to an equilibrium, with or without NFDS (for example, γ = 0 or −0.5; dashed and solid lines respectively), using blue and green lines to distinguish allele frequencies in habitats A and B, respectively. When NFDS is acting (solid lines), population divergence is much less pronounced than without NFDS, for a given strength of selection and migration. b–d show the equilibrium allele frequency difference (Δp, vertical axis) as a function of the migration rate, m, and the strength of NFDS, γ, for three strengths of DS, s = 0.001, 0.1 and 0.5 (b, c and d, respectively). This figure shows us the well known tendency for migration to constrain divergence (Δp declines as function of m). However, our addition of NFDS reveals a comparable constraint on divergence as a result of NFDS (Δp is smaller for more negative values of γ, indicating that NFDS constrains divergence). There is also an interaction between m and γ, which reflects the fact that the constraining effect of NFDS is most pronounced when migration rates are low, because the migrants are present but rare enough to benefit strongly from NFDS. At higher immigration rates, immigrants become increasingly common and their frequency-dependent advantage is reduced. We used multiple regression to measure the relative effects of m, s, and γ, and the m × γ interaction on Δp. Within the parameter space that we examined, selection has the strongest effect on final allele frequency divergence (55.9% of variance explained), migration is next strongest (20.8% of variance), and NFDS explains 5.8% of variance with an additional 5.7% of variance attributed to a γ × m interaction. Linear regression coefficients indicate that Δp declines by 0.050 per 0.1 unit of migration (a linear approximation of a nonlinear trend), whereas Δp declines by 0.022 for each 0.1 of γ below 0. We infer that the effect of γ (NFDS) is roughly 1/3 as strong as the effect of migration, but that these variables strongly interact. Thus, it is clear from our simulations that NFDS constrains population divergence by amplifying the effective migration rate. Simulation methods: we used a simple population genetic model to evaluate the relative effects of, and synergistic interaction between, migration, frequency-dependent selection, and DS. We used discrete-time deterministic numerical simulations to model the migration–selection balance between two discrete habitats A and B. Each habitat contained a haploid population with a polymorphic locus (alleles a and b) with allele frequencies p(a) + p(b) = 1. Each population was kept at a constant and effectively infinite population size so that genetic drift had no effect, and there were no demographic source–sink dynamics. In habitat A, the fitnesses of the two alleles were wa,A = 1.0 and wb,A = 1 − s, where s is the strength of selection against immigrants. Fitnesses were reversed in habitat B (wa,B = 1 − s and wb,B = 1). This symmetric DS was frequency-independent. Divergence in response to this selection was undermined by migration. Every generation, a fraction m of individuals in each habitat migrated to the other habitat as juveniles (before selection acts). Within each habitat, a fraction 1 − m of the residents did not disperse. To incorporate frequency-dependent selection, we adjusted each genotype’s fitness (after migration) to account for its relative abundance. Specifically, the frequency-dependent fitness of each allele i in habitat j was:  where γ dictates the strength and direction of frequency dependence (γ = 0 imparts no frequency dependence, γ < 0 imparts increasingly strong negative frequency dependence, and positive frequency dependence occurs when γ > 0; this is subject to the usual constraint that 0 ≤ p(i) ≤ 1.0). We focused exclusively on γ < 0 for our simulations, as we were interested specifically in negative frequency dependence. Note that this is merely a heuristic model to generate negative frequency-dependence to illustrate our point, and is not tailored to reflect a specific biological process. We then allowed selection to act on this population using the frequency-dependent fitness within each habitat. The frequency of allele a in the next generation in habitat j was:
where γ dictates the strength and direction of frequency dependence (γ = 0 imparts no frequency dependence, γ < 0 imparts increasingly strong negative frequency dependence, and positive frequency dependence occurs when γ > 0; this is subject to the usual constraint that 0 ≤ p(i) ≤ 1.0). We focused exclusively on γ < 0 for our simulations, as we were interested specifically in negative frequency dependence. Note that this is merely a heuristic model to generate negative frequency-dependence to illustrate our point, and is not tailored to reflect a specific biological process. We then allowed selection to act on this population using the frequency-dependent fitness within each habitat. The frequency of allele a in the next generation in habitat j was:  To examine the dynamics of this model, we initiated both populations with allele frequencies of 0.5, and iterated through multiple generations, with each generation containing a bout of migration, then frequency-dependent adjustment to fitness, then selection. We ran each simulation until the allele frequency reached an equilibrium in each habitat. For each simulation run we recorded the ending allele frequency difference between the habitats, ΔP = pA(a) – pB(a). Larger values of Δp denote more substantial genetic divergence between the populations. We repeated this simulation for a fully factorial combination of values of s (0.001, 0.005, 0.01, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5), m (0.001 to 0.5 in increments of 0.001), and γ (0 to 1 in increments of 0.001).
To examine the dynamics of this model, we initiated both populations with allele frequencies of 0.5, and iterated through multiple generations, with each generation containing a bout of migration, then frequency-dependent adjustment to fitness, then selection. We ran each simulation until the allele frequency reached an equilibrium in each habitat. For each simulation run we recorded the ending allele frequency difference between the habitats, ΔP = pA(a) – pB(a). Larger values of Δp denote more substantial genetic divergence between the populations. We repeated this simulation for a fully factorial combination of values of s (0.001, 0.005, 0.01, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5), m (0.001 to 0.5 in increments of 0.001), and γ (0 to 1 in increments of 0.001).
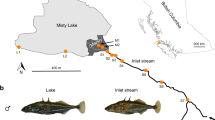
Extended Data Figure 2 A schematic diagram of our experimental design.
We constructed 40 cages in Roberts Lake, Vancouver Island, British Columbia, and 40 cages in the adjoining stream that drains out of the lake. We captured 120 wild-caught lake stickleback (indicated by blue shading), and 120 wild-caught stream stickleback (green shading). These were split evenly between lake and stream cages (60 per source and destination combination). Arrows with dashed perimeters indicate immigrants, solid perimeter arrows indicate residents. Within each habitat, we factorially manipulated the relative abundance of resident and immigrant fish (1:2 or 2:1 ratio; 20 cages per habitat per ratio).
Extended Data Figure 3 The main effect of minority versus majority status on stickleback survival (replotted from Fig. 1), after accounting for origin and destination.
We calculated the residuals from a binomial general linear mixed model regressing survival on fish origin and destination fixed effects with a random effect of cage. Here, we plot these residuals as a function of fish minority/majority status, showing residuals for all fish (open circles) and minority/majority means (filled circles) with ±1 standard error bars. A slight vertical and horizontal jitter has been added to distinguish otherwise overlapping points. Residual survival is significantly higher for minority ecotype fish (Wilcox rank sum test W = 7656, P = 0.0132).
Extended Data Figure 4 Both fish origin and transplant destination affect the identity and abundance of parasites infecting surviving fish.
Fish destination explains relatively more variance in parasite community composition. We used counts of each parasite in each fish to calculate Euclidean NMDS scores (using the metaMDS package in R). Here we plot individuals’ first and second NMDS axis scores. Individual points are colour-coded by origin (green for stream; blue for lake) and symbols denote destination (circle for lake; triangle for stream) as described in Fig. 1. Larger symbols indicate group means with one standard error bars. We subjected the two leading NMDS axes to a multivariate analysis of covariance (MANOVA) to test for effects of fish origin (Pillai’s trace = 0.086, P = 0.0015), destination (Pillai’s trace = 0.163, P = 0.0000029), and their interaction (Pillai’s trace = 0.008, P = 0.555). Fish with positive values of NMDS axis 1 carried more Neoechinorhynchus parasites but had fewer blackspot infections. Fish with positive values of NMDS axis 2 carried more Diplostomum but fewer Eustrongylides and Thersitina. NMDS axis 2 is primarily responsible for both the origin and destination effects of our transplant experiment: stream natives in stream cages had the highest NMDS axis 2 score, whereas lake fish in lake cages had the lowest NMDS axis 2 score. Non-native fish (transferred from lake to stream or vice versa) were intermediate between these extremes and not significantly different from each other. Thus, fish transferred between habitats converged partially on the parasite community of the native fish of their new habitat. Sample sizes are the number of survivors listed in Table 1.
Extended Data Figure 5 Lake versus stream divergence in MHC IIb genotypes.
Each unique exon 2 amino acid sequence (‘allele’) is plotted as a point, showing its prevalence in the lake-origin fish used in our transplant experiment (x axis) and stream-origin fish in the experiment (y axis). Because alleles can be distributed across MHC paralog copies, we calculate prevalence (the fraction of individuals carrying a given allele) rather than allele frequencies. Therefore the prevalences of all alleles sum to more than one. Alleles showing a significant lake versus stream difference in prevalence (binomial GLM, P < 0.05) are enlarged and coloured to indicate the habitat in which the allele is more common (blue for lake; green for stream). The diagonal line shows where allele frequencies are identical between the populations. Sample sizes of fish are 120 lake and 120 stream natives (Table 1).
Extended Data Figure 6 Simulations to evaluate the outcome of population divergence given both NFDS and directional selection (for example, higher fitness of stream fish everywhere).
As in Extended Data Fig. 1, here we plot the temporal dynamics of allele frequency in habitat A (green lines) and B (blue lines), with and without negative frequency dependence (solid and dashed lines respectively). Unlike in Extended Data Fig. 1, here we consider the evolutionary effects of symmetric migration (a, d and g), asymmetric migration (b, e and h) and biased migration (c, f and i) when there is directional selection (a–f) or DS (g–i). a–c illustrate the potential for initially divergent populations to remain at least partly differentiated despite directional selection. d–f illustrate the potential for initially similar populations to become genetically divergent despite directional selection. g–i illustrate the evolutionary dynamics for the same phenomena but when there is DS (as in Extended Data Fig. 1). These simulations are not meant to be an exhaustive analysis, but rather provide examples of possible evolutionary outcomes. In particular, the simulations are intended to demonstrate that lake–stream genetic divergence is plausible despite the directional selection favouring stream fish in both habitats. We repeated the simulations outlined in Extended Data Fig. 1 (R code is provided in the Supplementary Information), with the following modifications. (i) We compared the effects of DS (as in Extended Data Fig. 1) versus directional selection. For the directional selection we used fitness values directly drawn from Table 1, standardized to a maximum fitness of 1 within each habitat. That is, assuming the stream represents habitat A, the fitness of allele i in habitat j, wij, is wa,A = 1.0, wa,B = 1.0, wb,A = 0.74 and wb,B = 0.37. (ii) We introduced asymmetric migration (for instance, caused by water flow). We assumed that the emigration rate of the fitter ecotype mA→B is some fraction f of the reverse migration rate mB→A, so that the reciprocal migration rates can be expressed as (f × m, m). When f = 0, the universally fittest ecotype (for example, stream fish) cannot invade the habitat occupied by the less fit ecotype (for example, the lake), unless it is already present at non-zero frequency. (iii) We considered the case of biased movement. We assumed that allele a prefers to remain in habitat A, and allele b prefers to remain in habitat B. We therefore set their migration rates to zero. In contrast, the mismatched allele (b in habitat A; a in habitat B) emigrates at a rate m which we assume to be symmetric. For instance, in habitat A migration changes the frequency of allele a from pA to:  The parameter value sets used to illustrate these possible outcomes are illustrated in Extended Data Fig. 6. In panel a, γ = −0.6, m = 0.01, pA = 1.0, pB = 0.0, and selection coefficients are from Table 1. Panel b is the same as a except that migration is zero in the upstream (habitat A to habitat B) direction. In panel c, pA = 0.8 and pB = 0.2, the better to visualize complete fixation in the frequency-independent case, and m = 0.65, indicating that mismatched genotypes actively rather than randomly (m = 0.5) switch habitats. Panels d–f are the same as panels a–c but with different starting allele frequencies (pA = 0.55 and pB = 0.45) to illustrate that the equilibrium results in panels a and c are independent of initial conditions. However, evolution with asymmetric migration is highly sensitive to initial conditions because allele a will sweep to fixation within habitat B as long as it is present initially, whether or not there is migration. Panels g–i are the same as panels a–c but assuming symmetric DS (as in Extended Data Fig. 1) to examine the effects of asymmetric migration (h) and biased migration (i). For all three scenarios with DS, NFDS acts to undermine genetic divergence between the habitats (as in Extended Data Fig. 1). h, However, asymmetric migration means that the sink population is more polymorphic than the source population. i, Genotype-dependent dispersal enhances overall divergence between populations, as previously described38. As one would expect, the directional selection documented in Table 1 leads to rapid fixation of the ‘stream’ allele a in both habitats (a, dashed lines). However, the addition of NFDS (solid lines) leads to a counter-intuitive result: population divergence. Populations that would collapse to a single genotype (all p(a) = 1.0, dashed lines), instead maintain modest genetic differences when NFDS is added (the solid blue and green lines diverge; a). This result is easily explained: NFDS tends to maintain polymorphism within populations. For the particular parameter values chosen to illustrate this point (γ = −0.6, m = 0.01), NFDS maintains both alleles a and b in both habitats. The equilibrium for this stable polymorphism is higher for the blue line (habitat B) than the green line (habitat A) because NFDS must overcome stronger selection against allele b in habitat B. The equilibrium is insensitive to initial conditions (such as starting allele frequencies; d). This simulation thus points out that NFDS promotes polymorphism within populations (as is well known), but when these equilibrium allele frequencies differ between populations NFDS can sustain between-population genetic differences when they would not otherwise occur. This is an important point for our empirical system, because it suggests that strong NFDS could explain the persistence of allele frequency differences between lake and stream fish despite directional selection favouring one ecotype over the other. NFDS might thus resolve the conundrum posed in Supplementary Information section 1, subsection 3: why does lake–stream divergence persist despite widespread directional selection rather than the expected DS? When we add both directional selection and asymmetric migration (b), several results can occur. If we start with completely fixed differences between populations, then population differences persist despite directional selection. Even though allele a is favoured in both habitats in this simulation, it is initially absent in habitat B (blue, ‘lake’) and never arrives because migration is strictly directional from habitat B to A. Allele a fixes in habitat A (where it is favoured), and never makes it to habitat B (where a is also favoured). However, if we relax the initial conditions even slightly (non-fixed differences at the start, or weak upstream migration), then population divergence rapidly collapses as allele a fixes in both habitats either by a selective sweep within habitat A and B separately, or by immigration into habitat B followed by a sweep. Adding NFDS to the strict initial conditions (solid lines in b), allele a remains absent in habitat B because it is initially absent and there is no immigration. However, emigration from habitat B into A introduces allele b into the latter population, where it is maintained by NFDS despite frequency-independent directional selection (green line, whose equilibrium outcome matches the result in a). In the more relaxed initial conditions, the combination of directional selection, NFDS, and moderately asymmetric migration results in an outcome that looks very much like that in a. Next, we consider the case of biased dispersal in which allele a exhibits philopatry for habitat A, and allele b prefers habitat B. As described elsewhere38, this genotype-dependent dispersal facilitates population divergence (for example, divergence is greater in panels c, f and i than in panels a, d and g. Notably, this is possible even when allele a is favoured everywhere by selection (c). This is plausible if, for example, stream fish evolved to prefer the stream where they are fittest (Table 1), and lake fish prefer the lake as a refuge from stream fish. However, incorporating NFDS can prevent fixation of the philopatric types in one habitat (as shown in c) or in both habitats (not shown), depending on parameter values. In conclusion, these simulations confirm that there are multiple mechanisms that can explain the persistent divergence between lake and stream stickleback, even if the populations experience persistent directional rather than DS. The top row of figures here provides examples of allele-frequency differences arising from NFDS (a), asymmetric migration (b), and biased migration of genotypes (c). Of these, the most intriguing is the role of NFDS in generating stable allele frequency differences between populations (albeit not fixed differences).
The parameter value sets used to illustrate these possible outcomes are illustrated in Extended Data Fig. 6. In panel a, γ = −0.6, m = 0.01, pA = 1.0, pB = 0.0, and selection coefficients are from Table 1. Panel b is the same as a except that migration is zero in the upstream (habitat A to habitat B) direction. In panel c, pA = 0.8 and pB = 0.2, the better to visualize complete fixation in the frequency-independent case, and m = 0.65, indicating that mismatched genotypes actively rather than randomly (m = 0.5) switch habitats. Panels d–f are the same as panels a–c but with different starting allele frequencies (pA = 0.55 and pB = 0.45) to illustrate that the equilibrium results in panels a and c are independent of initial conditions. However, evolution with asymmetric migration is highly sensitive to initial conditions because allele a will sweep to fixation within habitat B as long as it is present initially, whether or not there is migration. Panels g–i are the same as panels a–c but assuming symmetric DS (as in Extended Data Fig. 1) to examine the effects of asymmetric migration (h) and biased migration (i). For all three scenarios with DS, NFDS acts to undermine genetic divergence between the habitats (as in Extended Data Fig. 1). h, However, asymmetric migration means that the sink population is more polymorphic than the source population. i, Genotype-dependent dispersal enhances overall divergence between populations, as previously described38. As one would expect, the directional selection documented in Table 1 leads to rapid fixation of the ‘stream’ allele a in both habitats (a, dashed lines). However, the addition of NFDS (solid lines) leads to a counter-intuitive result: population divergence. Populations that would collapse to a single genotype (all p(a) = 1.0, dashed lines), instead maintain modest genetic differences when NFDS is added (the solid blue and green lines diverge; a). This result is easily explained: NFDS tends to maintain polymorphism within populations. For the particular parameter values chosen to illustrate this point (γ = −0.6, m = 0.01), NFDS maintains both alleles a and b in both habitats. The equilibrium for this stable polymorphism is higher for the blue line (habitat B) than the green line (habitat A) because NFDS must overcome stronger selection against allele b in habitat B. The equilibrium is insensitive to initial conditions (such as starting allele frequencies; d). This simulation thus points out that NFDS promotes polymorphism within populations (as is well known), but when these equilibrium allele frequencies differ between populations NFDS can sustain between-population genetic differences when they would not otherwise occur. This is an important point for our empirical system, because it suggests that strong NFDS could explain the persistence of allele frequency differences between lake and stream fish despite directional selection favouring one ecotype over the other. NFDS might thus resolve the conundrum posed in Supplementary Information section 1, subsection 3: why does lake–stream divergence persist despite widespread directional selection rather than the expected DS? When we add both directional selection and asymmetric migration (b), several results can occur. If we start with completely fixed differences between populations, then population differences persist despite directional selection. Even though allele a is favoured in both habitats in this simulation, it is initially absent in habitat B (blue, ‘lake’) and never arrives because migration is strictly directional from habitat B to A. Allele a fixes in habitat A (where it is favoured), and never makes it to habitat B (where a is also favoured). However, if we relax the initial conditions even slightly (non-fixed differences at the start, or weak upstream migration), then population divergence rapidly collapses as allele a fixes in both habitats either by a selective sweep within habitat A and B separately, or by immigration into habitat B followed by a sweep. Adding NFDS to the strict initial conditions (solid lines in b), allele a remains absent in habitat B because it is initially absent and there is no immigration. However, emigration from habitat B into A introduces allele b into the latter population, where it is maintained by NFDS despite frequency-independent directional selection (green line, whose equilibrium outcome matches the result in a). In the more relaxed initial conditions, the combination of directional selection, NFDS, and moderately asymmetric migration results in an outcome that looks very much like that in a. Next, we consider the case of biased dispersal in which allele a exhibits philopatry for habitat A, and allele b prefers habitat B. As described elsewhere38, this genotype-dependent dispersal facilitates population divergence (for example, divergence is greater in panels c, f and i than in panels a, d and g. Notably, this is possible even when allele a is favoured everywhere by selection (c). This is plausible if, for example, stream fish evolved to prefer the stream where they are fittest (Table 1), and lake fish prefer the lake as a refuge from stream fish. However, incorporating NFDS can prevent fixation of the philopatric types in one habitat (as shown in c) or in both habitats (not shown), depending on parameter values. In conclusion, these simulations confirm that there are multiple mechanisms that can explain the persistent divergence between lake and stream stickleback, even if the populations experience persistent directional rather than DS. The top row of figures here provides examples of allele-frequency differences arising from NFDS (a), asymmetric migration (b), and biased migration of genotypes (c). Of these, the most intriguing is the role of NFDS in generating stable allele frequency differences between populations (albeit not fixed differences).
Extended Data Figure 7 NFDS can suppress or enhance population divergence.
a, When the populations in habitats A and B are subject to DS (as in Extended Data Fig. 1) and NFDS is absent (γ = 0), equilibrium allele frequency divergence is substantial and reflects the migration–selection balance. Stronger NFDS (more negative γ) reduces this equilibrium between-habitat divergence. b, When there is directional selection and no NFDS (γ = 0) the populations will fail to diverge because a single allele fixes in both habitats (see Extended Data Figs 1 and 6). Introducing NFDS (γ < 0) facilitates allele frequency difference between populations as long as selection is not identical in both habitats (for example, here wa,A = 1.0, wa,B = 1.0, wb,A = 0.8 and wb,B = 0.9). This is because NFDS favours different polymorphic equilibria in the two habitats, generating allele frequency differences (see Fig. 1a and Extended Data Fig. 6). However as NFDS strengthens (γ << 0) the effect of unequal directional selection becomes comparatively weak and the populations’ equilibrium allele frequencies converge again. The result is that there is an intermediate level of NFDS that can cause population genetic differences despite directional selection (b), when NFDS might otherwise undermine population genetic differences arising from DS (a).
Supplementary information
Supplementary Information
This file contains Supplementary Text and References. (PDF 234 kb)
Supplementary Data
This zipped file contains the source code for simulations in Extended Data Figures 1, 8 and 9. (ZIP 6 kb)
Rights and permissions
About this article
Cite this article
Bolnick, D., Stutz, W. Frequency dependence limits divergent evolution by favouring rare immigrants over residents. Nature 546, 285–288 (2017). https://doi.org/10.1038/nature22351
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature22351
This article is cited by
-
Immunogenetic-pathogen networks shrink in Tome’s spiny rat, a generalist rodent inhabiting disturbed landscapes
Communications Biology (2024)
-
Increasing our ability to predict contemporary evolution
Nature Communications (2020)
-
Host–parasite co-evolution and its genomic signature
Nature Reviews Genetics (2020)
-
Differences can hold populations together
Nature (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.