Abstract
Effective adaptive immune responses require a large repertoire of naive T cells that migrate throughout the body, rapidly identifying almost any foreign peptide1. Because the production of T cells declines with age, naive T cells must be long-lived2. However, it remains unclear how naive T cells survive for years while constantly travelling. The chemoattractant sphingosine 1-phosphate (S1P) guides T cell circulation among secondary lymphoid organs, including spleen, lymph nodes and Peyer’s patches, where T cells search for antigens. The concentration of S1P is higher in circulatory fluids than in lymphoid organs, and the S1P1 receptor (S1P1R) directs the exit of T cells from the spleen into blood, and from lymph nodes and Peyer’s patches into lymph3. Here we show that S1P is essential not only for the circulation of naive T cells, but also for their survival. Using transgenic mouse models, we demonstrate that lymphatic endothelial cells support the survival of T cells by secreting S1P via the transporter SPNS2, that this S1P signals through S1P1R on T cells, and that the requirement for S1P1R is independent of the established role of the receptor in guiding exit from lymph nodes. S1P signalling maintains the mitochondrial content of naive T cells, providing cells with the energy to continue their constant migration. The S1P signalling pathway is being targeted therapeutically to inhibit autoreactive T cell trafficking, and these findings suggest that it may be possible simultaneously to target autoreactive or malignant cell survival4.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Jenkins, M. K., Chu, H. H., McLachlan, J. B. & Moon, J. J. On the composition of the preimmune repertoire of T cells specific for peptide–major histocompatibility complex ligands. Annu. Rev. Immunol. 28, 275–294 (2010)
Boehm, T. & Swann, J. B. Thymus involution and regeneration: two sides of the same coin? Nat. Rev. Immunol. 13, 831–838 (2013)
Cyster, J. G. & Schwab, S. R. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 30, 69–94 (2012)
Proia, R. L. & Hla, T. Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J. Clin. Invest. 125, 1379–1387 (2015)
Mendoza, A. et al. The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate. Cell Rep. 2, 1104–1110 (2012)
Fukuhara, S. et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Invest. 122, 1416–1426 (2012)
Hisano, Y., Kobayashi, N., Yamaguchi, A. & Nishi, T. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS One 7, e38941 (2012)
Nagahashi, M. et al. Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J. 27, 1001–1011 (2013)
Nijnik, A. et al. The role of sphingosine-1-phosphate transporter Spns2 in immune system function. J. Immunol. 189, 102–111 (2012)
Pham, T.H. M. et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 207, 17–27 (2010)
Sentman, C. L., Shutter, J. R., Hockenbery, D., Kanagawa, O. & Korsmeyer, S. J. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell 67, 879–888 (1991)
Fang, V. et al. Gradients of the signaling lipid S1P in lymph nodes position natural killer cells and regulate their interferon-γ response. Nat. Immunol. 18, 15–25 (2017)
Schwab, S. R. et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309, 1735–1739 (2005)
Liu, C. H. et al. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol. Biol. Cell 10, 1179–1190 (1999)
Matloubian, M. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004)
Allende, M. L., Dreier, J. L., Mandala, S. & Proia, R. L. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J. Biol. Chem. 279, 15396–15401 (2004)
Takada, K. & Jameson, S. C. Naive T cell homeostasis: from awareness of space to a sense of place. Nat. Rev. Immunol. 9, 823–832 (2009)
Chang, J. E. & Turley, S. J. Stromal infrastructure of the lymph node and coordination of immunity. Trends Immunol. 36, 30–39 (2015)
Surh, C. D. & Sprent, J. Homeostasis of naive and memory T cells. Immunity 29, 848–862 (2008)
Link, A. et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat. Immunol. 8, 1255–1265 (2007)
Milasta, S. et al. Apoptosis-inducing-factor-dependent mitochondrial function is required for T cell but not B cell function. Immunity 44, 88–102 (2016)
Chang, C. H. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 (2013)
Pickrell, A. M. & Youle, R. J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85, 257–273 (2015)
Liu, Y. et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 106, 951–961 (2000)
Mizugishi, K. et al. Essential role for sphingosine kinases in neural and vascular development. Mol. Cell. Biol. 25, 11113–11121 (2005)
Liu, G. et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat. Immunol. 10, 769–777 (2009)
Allende, M. L., Yamashita, T. & Proia, R. L. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood 102, 3665–3667 (2003)
Allende, M. L. et al. Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J. Biol. Chem. 286, 7348–7358 (2011)
Ruzankina, Y. et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 1, 113–126 (2007)
Schaefer, B. C., Schaefer, M. L., Kappler, J. W., Marrack, P. & Kedl, R. M. Observation of antigen-dependent CD8+ T-cell/ dendritic cell interactions in vivo. Cell. Immunol. 214, 110–122 (2001)
Madsen, L. et al. Mice lacking all conventional MHC class II genes. Proc. Natl Acad. Sci. USA 96, 10338–10343 (1999)
Sudo, T. et al. Expression and function of the interleukin 7 receptor in murine lymphocytes. Proc. Natl Acad. Sci. USA 90, 9125–9129 (1993)
Acknowledgements
We thank members of the Schwab and A. Sfeir laboratories for discussions, T. Trimarchi for help analysing RNA-seq data, R. Proia for S1pr4−/− mice, S. Milasta for advice on glucose/galactose activation, and A. Sfeir for critical reading of the manuscript. This work was supported by National Institutes of Health (NIH) grants R01 AI085166 and AI123308 to S.R.S.; NIH R01 CA206005, Tisch Cancer Institute Cancer Center Support Grant (P30 CA196521), an American Cancer Society Research Scholar Grant, and a Leukemia & Lymphoma Society Career Development Award to J.E.C.; US National Science Foundation CAREER grant 1054964 and NCI R01 CA194547 to O.E.; NIH R01 HL089934 to T.H.; NIH R37 AI43542 and Wellcome Trust and Kennedy Trust for Rheumatology Research PRF 100262Z/12/Z to J.M. and M.L.D.; and NIH T32 AI100853 to A.M., V.F. and C.C.
Author information
Authors and Affiliations
Contributions
A.M. designed and conducted all experiments, analysed data, and wrote the manuscript; V.F. performed imaging and image quantification; C.C. performed preliminary experiments with DOP; M.S. performed mitochondrial function experiments; A.V. performed RNA-seq analysis; J.M. and V.S.C. performed imaging; M.L.D., T.H., O.E. and J.E.C. designed experiments and interpreted data; S.R.S. designed experiments, interpreted data, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks N. Chandel, Y. A. Hannun and R. L. Proia for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
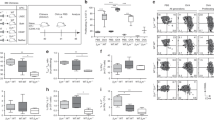
Extended Data Figure 1 SPNS2 is required in lymphatic endothelial cells for peripheral T cell circulation.
a, Diagram of distribution of naive T cells in the periphery of Spns2Δ mice and controls. Spns2Δ mice have normal plasma S1P and lymphocytes exit from the spleen into blood as usual, but in Spns2Δ mice lymph S1P is lost and egress from LN is blocked. Over time, this leads to a redistribution of T cells from the spleen to the LN and a loss of circulating cells, because any cell that leaves the spleen and enters a lymph node is trapped. b, Representative surface S1P1R on naive CD4+ T cells in the blood and spleen (top) and in the lymph and LN (bottom) of a Spns2Δ mouse and its littermate control. Surface S1P1R on T cells is internalized upon binding S1P. Hence the lower S1P1R on the surface of T cells in blood than in spleen, in both Spns2Δ and control animals, indicates that T cells sense more S1P in blood than in spleen. S1P1R is also lower on the surface of T cells in lymph than LN of control mice. But in Spns2Δ mice, S1P1R is equally high on T cells in lymph and LN, suggesting that the gradient that directs T cell exit from LN has been ablated. c–h, T cell distribution in Spns2Δ mice and littermate controls. c, d, Percentage of total peripheral naive CD4 (c) and CD8 (d) T cells in the spleen and LN. Total peripheral lymphocytes are defined as those in spleen and a subset of LN (brachial, axillary, inguinal and mesenteric); blood and lymph make a negligible contribution. e, f, Number of naive CD4 (e) and CD8 (f) T cells in blood and lymph. g, Total number of naive CD8 T cells in the periphery. h, Number of naive CD8 T cells in the spleen and LN. Data in b–h are representative of or pool 8 pairs of mice analysed in 8 experiments. i, j, Frequency of PI+ (i) and apoptotic (j) naive CD8 T cells in LN of Spns2Δ mice and littermate controls. 6 pairs of mice analysed in 6 experiments. k–n, Frequency of apoptotic (k, m) and PI+ (l, n) naive CD4 T cells (k, l) and CD8 T cells (m, n) in spleen of Spns2Δ mice and littermate controls. 5 pairs of mice analysed in 5 experiments. There is no statistically significant difference in the death of naive CD4 or CD8 T cells in the spleen of Spns2Δ mice compared to littermate controls, although there seems to be a trend towards increased death in Spns2Δ mice. Normal T cell survival in the spleen of Spns2Δ mice is consistent with normal blood S1P and normal S1P production by blood vessel endothelial cells lining the marginal sinus, which probably maintain normal S1P1R signalling in T cells in the spleen. We hypothesize that the few T cells able to leave the LN of Spns2Δ mice are relatively healthy, and stabilized while they are in the spleen. However, we hesitate to overinterpret these results because very few T cells remain in the spleen of Spns2Δ animals, and they may be different in a way that is not captured by gating on CD62LhiCD44lo T cells. o, p, Congenically labelled lymphocytes from BCL2-transgenic and wild-type littermate donors were co-transferred (1:1 by naive CD4 T cell counts) to Spns2Δ mice and littermate controls. Number of wild-type or BCL2-transgenic naive CD8 T cells recovered in spleen and LN 21 days after transfer. 9 pairs of mice analysed in 3 experiments. Lines indicate mean. *P < 0.05, **P < 0.01, ***P < 0.001 ****P < 0.0001, unpaired two-tailed t-test.
Extended Data Figure 2 Spns2 deletion in lymphatic endothelial cells does not block thymic egress, and does not induce substantial accumulation of naive T cells in tissues other than LN and spleen.
A reduction in the efficiency of T cell exit from the thymus is reflected in an accumulation of mature (CD69lowCD62Lhi) single positive (SP) T cells. a, Expression of CD4 and CD8 by total thymocytes (top), and expression of CD69 and CD62L by CD4 single-positive thymocytes (bottom), from a representative Spns2Δ mouse and littermate control. b, Percentage mature of total CD4 single-positive T cells and CD8 single-positive T cells in Spns2Δ mice and littermate controls. c, Number of mature single-positive CD4 and CD8 thymocytes in Spns2Δ mice and littermate controls. Graphs in b and c compile 5 pairs of mice analysed in 5 experiments. d, e, Number of naive CD4 and CD8 T cells in the Peyer’s patches (d) and bone marrow (e) of Spns2Δ mice and littermate controls. f–h, Number of CD4 and CD8 T cells in liver (f), lungs (g) and small intestine lamina propria (h) of Spns2Δ mice and littermate controls. Interestingly, T cells do accumulate in the Peyer’s patches of Spns2Δ mice, but the numbers account for only around 5% of the missing cells (there were approximately 2.1 × 107 fewer naive CD4 T cells in the combined spleen, mesenteric, inguinal, brachial and axillary LN of Spns2Δ mice compared to controls, and 9.6 × 105 more naive CD4 T cells in the Peyer’s patches of Spns2Δ mice compared to controls). Graph in d pools 8 pairs of mice analysed in 8 experiments, and graphs in e–h pool 3 pairs of mice analysed in 3 experiments. Lines indicate mean. †P = 0.07 (e), †P = 0.095 (h), *P < 0.05, **P < 0.01, unpaired two-tailed t-test.
Extended Data Figure 3 Loss of naive T cells in Spns2Δ mice is not due to conversion to another cell type.
To assess whether T cells in Spns2Δ mice lose quiescence and die of activation-induced apoptosis, we assessed CD44 expression, cell size and incorporation of BrdU. a, Expression of CD44 and CD62L on CD4 T cells in LN of representative Spns2Δ and littermate control mice. b, Percentage CD44hi of total CD4 and percentage CD44hi of total CD8 T cells in LN. c, Number of CD44hiCD4+ T cells and CD44hiCD8+ T cells in LN. Data in b and c pool 9 pairs of mice analysed in 9 experiments. d, Forward scatter area (FSC-A) of naive CD4 T cells in LN of a representative Spns2Δ (shaded) and littermate control (black) mouse. e, Ratio of mean FSC-A of naive CD4 or CD8 T cells in the LN of a Spns2Δ mouse to mean FSC-A of naive CD4 or CD8 T cells in the LN of its littermate control. Data pool 16 pairs of mice analysed in 16 experiments. f, Mice were injected intraperitoneally with BrdU daily for 3 days. 24 h after the last injection, LN were collected and naive CD4 and CD8 T cells were analysed by flow cytometry for BrdU incorporation. Data pool 5 pairs of mice analysed in 5 experiments. g, Frequencies of induced regulatory T cells (iTreg) and natural regulatory T cells (nTreg) in the LN of Spns2Δ and littermate control mice. iTreg were defined as FOXP3+neuropilin− and nTreg were defined as FOXP3+neuropilin+. Data pool 3 pairs of mice analysed in 3 experiments. Lines indicate mean. †P = 0.051, *P < 0.05, ***P < 0.001, unpaired two-tailed t-test.
Extended Data Figure 4 Spns2 is required in Lyve1-cre-deleted radioresistant cells.
Hosts were lethally irradiated and reconstituted with bone marrow from the specified congenically marked donors. Mice were analysed at least 6 weeks after transplantation. a, Representative flow cytometry plot of surface S1P1R on T cells in lymph of the indicted chimaeras (left). Ratio of surface S1P1R mean fluorescence intensity (MFI) on naive T cells in the lymph of a Spns2Δ mouse reconstituted with wild-type bone marrow to surface S1P1R MFI on naive T cells in the lymph of a littermate control with wild-type bone marrow (right). Graph compiles 5 pairs of mice analysed in 5 experiments. b, Representative flow cytometry plot of surface S1P1R on T cells in lymph of the indicted chimaeras (left). Ratio of surface S1P1R MFI on naive T cells in the lymph of a wild-type mouse reconstituted with Spns2Δ bone marrow to surface S1P1R MFI on naive T cells in the lymph of a wild-type mouse reconstituted with littermate control bone marrow (right). Graph compiles 5 pairs of mice analysed in 5 experiments. c, Total number of donor-derived naive CD4 and CD8 T cells in the periphery (combined spleen and mesenteric, axillary, inguinal and brachial LN) of the indicated chimaeras. d, Number of donor-derived naive CD4 T cells in the lymph, blood, LN and spleen of the indicated chimaeras. e, Frequency of apoptotic donor-derived naive CD4 T cells in the LN of the indicated chimaeras. f, Number of donor-derived naive CD8 T cells in the lymph, blood, LN and spleen of the indicated chimaeras. g, Frequency of apoptotic donor-derived naive CD8 T cells in the LN of the indicated chimaeras. c–g compile 5 sets of mice (made using 2 pairs of Spns2Δ and control donors and 2 wild-type donors) analysed in 5 experiments. h, i, Reconstitution of macrophages in the LN of chimaeras. h, Gating scheme to assess reconstitution in bulk CD11b+ cells and CD11b+CD169+ sinus-lining macrophages. Representative plots for 4 sets of mice analysed in 4 experiments. i, Percentage of donor-derived CD11b+ and CD11b+CD169+ macrophages. The average percentage of donor-derived CD45+ haematopoietic cells was 92%, CD11b+ macrophages was 89%, and CD11b+CD169+ macrophages was 87%. Graphs compile 4 sets of mice (made using 2 pairs of Spns2Δ and control donors and 2 wild-type donors) analysed in 4 experiments. Lines indicate mean. †P = 0.061, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, unpaired two-tailed t-test.
Extended Data Figure 5 Naive T cell survival is dependent on S1P, but independent of circulation.
Spns2Δ mice and littermate controls were treated for 21 days with 30 mg l−1 DOP and 10 g l−1 sucrose, or sucrose alone, in the drinking water. a–c, The effect of DOP treatment on T cell exposure to extracellular S1P within the LN was assessed by measuring surface expression of S1P1R on naive T cells, which is inversely related to S1P levels. a, Representative flow cytometry plot of surface S1P1R on naive CD4 T cells. An FTY720-treated mouse was used as a negative control for S1P1R staining. b, Ratio of cell surface S1P1R MFI on naive CD4 T cells from LN of the indicated mice to cell surface S1P1R MFI on naive CD4 T cells from LN of sucrose-treated control mice. 5 groups of mice analysed in 5 experiments. c, Ratio of cell surface S1P1R MFI on naive CD8 T cells from LN of the indicated mice to cell surface S1P1R MFI on naive CD8 T cells from LN of sucrose-treated control mice. 4 groups of mice analysed in 4 experiments. d, e, Number of naive CD4 (d) or CD8 (e) T cells in the blood of Spns2Δ mice and littermate controls, with and without DOP treatment. 8 groups of mice analysed in 5 experiments. f, g, Frequency of PI+ (f) and apoptotic (g) naive CD8 T cells in the LN of Spns2Δ mice and littermate controls, with and without DOP treatment. 5 groups (control and Spns2Δ groups had a total of 6 mice, DOP-treated control and DOP-treated Spns2Δ groups had a total of 5 mice) analysed in 3 experiments. h, Congenically marked BCL2-transgenic and littermate wild-type lymphocytes (at a 1:1 ratio for naive CD4 T cell counts) were co-transferred to Spns2Δ and littermate control mice, with and without DOP treatment. 21 days after transfer, wild-type or BCL2-transgenic naive CD8 T cells recovered in LN were enumerated. Ratio of wild-type to BCL2-transgenic T cells is shown for control and DOP-treated Spns2Δ mice and littermate controls. 10 mice per group of recipients analysed in 4 experiments. Lines indicate mean. *P < 0.05, **P < 0.01, ***P < 0.001, unpaired two-tailed t-test.
Extended Data Figure 6 Naive T cell survival is not dependent on S1PR4 signalling.
Wild-type CD45.1+ mice were lethally irradiated and reconstituted with bone marrow from CD45.2+ S1pr4−/− or littermate control mice. Mice were analysed 6 weeks after bone marrow reconstitution. a, Percentage of semi-mature (CD69hiCD62Llo) and mature (CD69loCD62Lhi) single-positive CD4 or CD8 thymocytes in S1pr4−/− and control chimaeras. b, Number of semi-mature and mature single-positive CD4 and CD8 thymocytes. c–e, Number of naive CD4 and CD8 T cells in blood (c), spleen (d) and LN (e). LN quantified were brachial, axillary, inguinal and mesenteric. Two sets of S1pr4−/− and littermate control bone marrow donors were used. Data pool 5 pairs of chimaeras analysed in 3 experiments, all gated on CD45.2+ cells. Lines indicate mean. *P < 0.05, unpaired two-tailed t-test.
Extended Data Figure 7 Cell autonomous S1P1R signalling is required in naive CD8 T cells to inhibit apoptosis.
a, b, Adult S1pr1f/fUBC-creERT2 mice and littermate controls were thymectomized, treated with tamoxifen, and analysed 12 weeks later (tamoxifen-treated S1pr1f/fUBC-creERT2 mice are referred to as S1pr1Δ). a, Number of naive CD8 T cells in LN (mesenteric, axillary, inguinal and brachial) and spleen of S1pr1Δ mice and littermate controls. b, Frequency of apoptotic naive CD8 T cells in LN and spleen of S1pr1Δ mice and littermate controls. 5 pairs of mice analysed in 3 experiments. c, d, CD45.1+ mice were lethally irradiated and reconstituted with a mix of bone marrow from wild-type UBC-GFP mice and from either S1pr1f/fUBC-creERT2 CD45.2+ mice or littermate controls. The mice were thymectomized 6 weeks after reconstitution and treated with tamoxifen 4 weeks after surgery. c, Ratio of the number of S1pr1f/fUBC-creERT2 or littermate control naive CD8 T cells to the number of GFP+ naive CD8 T cells found in the blood before tamoxifen-induced S1pr1 deletion, and the same ratio in the blood, LN and spleen 24 weeks after tamoxifen treatment. 5 pairs of mice from 2 bone marrow donor sets (except control spleen, with 4 mice), analysed in 3 experiments. d, Frequency of apoptotic S1pr1Δ or littermate control naive CD8 T cells and GFP+ wild-type naive CD8 T cells. 10 pairs of mice from 3 bone marrow donor sets analysed in 5 experiments 12 or 24 weeks after tamoxifen treatment. e, CD45.1+ wild-type mice were irradiated and reconstituted with a 1:1 ratio of UBC-GFP wild-type bone marrow and either CD45.2+ S1pr1f/f or S1pr1f/f;UBC-creERT2 bone marrow. After 6 weeks, Cre activity was induced with 5 daily injections of tamoxifen (in both groups of mice). 5 days after the last dose of tamoxifen, naive GFP−CD45.2+ CD4 T cells were sorted from LN. Transcripts were quantified by RNA-seq, and differentially expressed genes (Benjamini–Hochberg adjusted P < 0.05) were analysed using ingenuity pathway analysis (Qiagen version 1.0). Differentially expressed transcripts that fell into the ‘Molecular and cellular functions’ category with the annotation ‘Cell death and survival’ are shown. Lines indicate mean. †P = 0.092, *P < 0.05, **P < 0.01, ****P < 0.0001, unpaired two-tailed t-test.
Extended Data Figure 8 DOP does not rescue cell death in S1pr1-deficient mice.
a–d, Frequency of apoptotic (a, c) and PI+ (b, d) naive CD4 (a, b) and naive CD8 (c, d) T cells in the LN of S1pr1Δ mice and littermate controls, with and without DOP treatment (30 mg l−1 DOP with 10 g l−1 sucrose in drinking water, or sucrose alone, for 3 weeks, as in Fig. 2). 6 groups of mice analysed in 4 experiments. Lines indicate mean. *P < 0.05, **P < 0.01, unpaired two-tailed t-test.
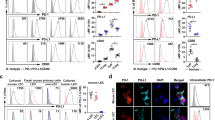
Extended Data Figure 9 Little evidence that S1P1R regulates access to IL-7 or self-peptide/MHC.
a, b, Sorted naive CD4 T cells (which do not express MHC class II) (a) and sorted naive CD8 T cells (b) from LN of S1pr1Δ and littermate control animals were cultured for 5 days in the indicated concentrations of IL-7. Frequency of PI+ cells measured by flow cytometry. Graph pools 9 experiments (a) or 6 experiments (b). c, Western blot of CD4 T cells from LN stimulated ex vivo with IL-7. Representative of 2 experiments. d, e, Immunofluorescence staining for the indicated markers in the lymph node T zone. FRC, fibroblastic reticular cells. Representative of 3 experiments. f, Representative cell surface IL-7Rα on naive CD4 T cells from LN. g, Ratio of IL-7Rα MFI on S1pr1Δ naive CD4 and CD8 T cells from LN compared to littermate controls. Compiles data from 4 pairs in 4 experiments. h, Representative Nur77 expression by naive CD4 T cells isolated from LN of S1pr1Δ and littermate control animals. Naive CD4 T cells transferred to MHCII-knockout (KO) recipients and activated CD4 T cells were used as negative and positive controls for Nur77 expression, respectively. i, Ratio of Nur77 MFI in naive T cells from LN of S1pr1Δ mice compared to littermate controls. Graph compiles 4 pairs of mice analysed in 4 experiments. j–m, S1pr1Δ and littermate control mice were treated with either IL-7Rα blocking or isotype control antibody, and analysed 5 days later. j, Efficacy of IL-7Rα blockade was assessed by staining for IL-7Rα (using the anti-IL-7Rα blocking antibody) on naive CD4 T cells from LN of mice treated with anti-IL-7Rα and isotype control. k, Efficacy of IL-7Rα blockade was measured by the ability of CD4 T cells from LN of anti-IL-7Rα-treated and isotype-control-treated mice to phosphorylate STAT5 in response to IL-7 ex vivo (5 min stimulation with the indicated concentrations of IL-7), measured by western blot. Representative of 3 experiments. l–m, Frequency of apoptotic naive CD4 (l) and CD8 (m) T cells in LN. 8 groups of mice analysed in 4 experiments. n, Sorted naive CD4 T cells from LN of S1pr1Δ animals and littermate controls were transferred to MHCII-deficient recipients. Cells in recipient LN were analysed 5 days later. 4 pairs in 4 experiments. We cannot exclude effects below our limit of detection, or the possibility that pre-existing defects in S1pr1Δ cells preserve the differences measured in our assays. Lines denote mean, error bars denote s.e.m. †P = 0.052, *P < 0.05, **P < 0.01, unpaired two-tailed t-test.
Extended Data Figure 10 Naive T cells require S1P1R signalling for maintenance of mitochondrial content and function.
a, Naive CD8 T cells from LN of S1pr1Δ mice and littermate controls were analysed by flow cytometry for total mitochondria (MitoTracker Deep Red FM) and functional mitochondria (MitoTracker Red CMX-Ros). Representative histograms and compiled ratios are shown for 4 pairs of mice analysed in 4 experiments. b, c, Frequency of apoptotic naive CD4 (b) or CD8 (c) T cells from LN of control, S1pr1Δ, control BCL2+, and S1pr1Δ BCL2+ mice. Compiles 3 sets of mice analysed in 3 experiments. d, e, Mitochondrial function was assayed ex vivo in sorted naive CD4 and CD8 T cells from LN of control BCL2+ and S1pr1Δ BCL2+ mice using XF Cell Mito Stress Test Kit and an XFe96 Extracellular Flux Analyzer (Agilent Technologies, formerly Seahorse). d, OCR of naive CD4 T cells from LN of control BCL2+ and S1pr1Δ BCL2+mice. Graph plots replicates from 1 experiment. Error bars show s.e.m. Data are representative of 2 pairs of mice analysed in 2 experiments. e, OCR of naive CD8 T cells from LN of control BCL2+ and S1pr1Δ BCL2+mice. Graph plots replicates from 1 experiment. Error bars show s.e.m. Data are representative of 2 pairs of mice analysed in 2 experiments. f, Ratio of average basal and maximal OCR of S1pr1Δ naive CD8 T cells from LN to the average basal and maximal OCR of littermate controls. Graph compiles 3 pairs of mice analysed in 3 experiments. Black dots indicate OCR ratios between S1pr1Δ mice and controls, grey dots indicate OCR ratios between S1pr1Δ;BCL2-transgenic mice and BCL2-transgenic controls. g–j, CellTrace Violet-labelled CD4 and CD8 T cells from LN of S1pr1Δ mice and littermate controls were activated with anti-CD3/CD28 and cultured in medium supplemented with glucose or galactose for 72 h. CellTrace Violet dilution for CD4 (g) and CD8 (h) T cells and total numbers of CD4 (i) and CD8 (j) T cells measured 72 h after activation. Graph compiles triplicate samples from 4 pairs of mice analysed in 4 experiments. k, Transcripts upregulated as part of the ER unfolded protein response—Eif2ak3 (PERK), Hspa5 (BIP), and Ddit3 (CHOP)—were measured by reverse transcription quantitative PCR (RT–qPCR) on sorted naive CD4 T cells from LN of S1pr1Δ and littermate control mice. Data compile 4 sets of cells sorted from 4 pairs of mice. Error bars denote s.e.m. l, Ratio of β-actin signal quantified by western blot to the number of CD4 T cells loaded per well from S1pr1Δ mice and littermate controls. Graph compiles 4 sets of samples from 4 pairs of mice analysed in 2 experiments (a subset of the samples in Fig. 4b). m, CD4 T cells isolated from LN were stimulated ex vivo with 1 μM S1P or vehicle for 3 h. AKT and S6 phosphorylation were assessed by western blot. Representative of 2 experiments. Lines denote mean, error bars denote s.e.m. †P = 0.053, *P < 0.05, **P < 0.01, unpaired two-tailed t-test.
Supplementary information
Supplementary Information
This file contains the ImageJ program to quantify co-localization between stains for mitochondria and ubiquitin and the uncropped blots. (PDF 288 kb)
Source data
Rights and permissions
About this article
Cite this article
Mendoza, A., Fang, V., Chen, C. et al. Lymphatic endothelial S1P promotes mitochondrial function and survival in naive T cells. Nature 546, 158–161 (2017). https://doi.org/10.1038/nature22352
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature22352
This article is cited by
-
Dysregulated cellular metabolism in atherosclerosis: mediators and therapeutic opportunities
Nature Metabolism (2024)
-
Functional roles of sphingolipids in immunity and their implication in disease
Experimental & Molecular Medicine (2023)
-
Mass cytometry analysis identifies T cell immune signature of aplastic anemia and predicts the response to cyclosporine
Annals of Hematology (2023)
-
Iron Deficiency Increases Phosphorylation of SP1 to Upregulate SPNS2 Expression in Hepatocellular Carcinoma
Biological Trace Element Research (2023)
-
The Sphingosine 1-Phosphate Axis: an Emerging Therapeutic Opportunity for Endometriosis
Reproductive Sciences (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.