Abstract
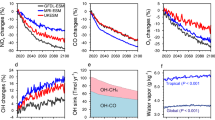
The abundance of tropospheric oxidants, such as ozone (O3) and hydroxyl (OH) and peroxy radicals (HO2 + RO2), determines the lifetimes of reduced trace gases such as methane and the production of particulate matter important for climate and human health. The response of tropospheric oxidants to climate change is poorly constrained owing to large uncertainties in the degree to which processes that influence oxidants may change with climate1 and owing to a lack of palaeo-records with which to constrain levels of atmospheric oxidants during past climate transitions2. At present, it is thought that temperature-dependent emissions of tropospheric O3 precursors and water vapour abundance determine the climate response of oxidants, resulting in lower tropospheric O3 in cold climates while HOx (= OH + HO2 + RO2) remains relatively buffered3. Here we report observations of oxygen-17 excess of nitrate (a proxy for the relative abundance of atmospheric O3 and HOx) from a Greenland ice core over the most recent glacial–interglacial cycle and for two Dansgaard–Oeschger events. We find that tropospheric oxidants are sensitive to climate change with an increase in the O3/HOx ratio in cold climates, the opposite of current expectations. We hypothesize that the observed increase in O3/HOx in cold climates is driven by enhanced stratosphere-to-troposphere transport of O3, and that reactive halogen chemistry is also enhanced in cold climates. Reactive halogens influence the oxidative capacity of the troposphere directly as oxidants themselves and indirectly4 via their influence on O3 and HOx. The strength of stratosphere-to-troposphere transport is largely controlled by the Brewer–Dobson circulation5, which may be enhanced in colder climates owing to a stronger meridional gradient of sea surface temperatures6, with implications for the response of tropospheric oxidants7 and stratospheric thermal and mass balance8. These two processes may represent important, yet relatively unexplored, climate feedback mechanisms during major climate transitions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Naik, V. et al. Preindustrial to present-day changes in tropospheric hydroxyl radical and methane lifetime from the Atmospheric Chemistry and Climate Model Intercomparison Project (ACCMIP). Atmos. Chem. Phys. 13, 5277–5298 (2013)
Alexander, B. & Mickley, L. Paleo-perspectives on potential future changes in the oxidative capacity of the atmosphere due to climate change and anthropogenic emissions. Curr. Pollution Rep. 1, 57–69 (2015)
Murray, L. T. et al. Factors controlling variability in the oxidative capacity of the troposphere since the Last Glacial Maximum. Atmos. Chem. Phys. 14, 3589–3622 (2014)
Schmidt, J. A. et al. Modeling the observed tropospheric BrO background: importance of multiphase chemistry and implications for ozone, OH, and mercury. J. Geophys. Res. 121, 11819–11835 (2016)
Holton, J. R. et al. Stratosphere-troposphere exchange. Rev. Geophys. 33, 403–439 (1995)
Rind, D., Lerner, J., McLinden, C. & Perlwitz, J. Stratospheric ozone during the Last Glacial Maximum. Geophys. Res. Lett. 36, L09712 (2009)
Hegglin, M. I. & Shepherd, T. G. Large climate-induced changes in ultraviolet index and stratosphere-to-troposphere ozone flux. Nat. Geosci. 2, 687–691 (2009)
Butchart, N. The Brewer-Dobson circulation. Rev. Geophys. 52, 157–184 (2014)
Alexander, B. et al. Quantifying atmospheric nitrate formation pathways based on a global model of the oxygen isotopic composition (Δ17O) of atmospheric nitrate. Atmos. Chem. Phys. 9, 5043–5056 (2009)
Savarino, J. et al. Isotopic composition of atmospheric nitrate in a tropical marine boundary layer. Proc. Natl Acad. Sci. USA 110, 17668–17673 (2013)
Morin, S., Savarino, J., Bekki, S., Gong, S. & Bottenheim, J. W. Signature of Arctic surface ozone depletion events in the isotope anomaly (Δ17O) of atmospheric nitrate. Atmos. Chem. Phys. 7, 1451–1469 (2007)
Alley, R. B. Wally was right: predictive ability of the North Atlantic “Conveyor Belt” hypothesis for abrupt climate change. Annu. Rev. Earth Planet. Sci. 35, 241–272 (2007)
Barrie, L. A., Bottenheim, J. W., Schnell, R. C., Crutzen, P. J. & Rasmussen, R. A. Ozone destruction and photochemical reactions at polar sunrise in the lower Arctic atmosphere. Nature 334, 138–141 (1988)
Rind, D., Chandler, M., Lonergan, P. & Lerner, J. Climate change and the middle atmosphere. 5. Paleostratosphere in cold and warm climates. J. Geophys. Res. 106, 20195–20212 (2001)
Lin, P. & Fu, Q. Changes in various branches of the Brewer–Dobson circulation from an ensemble of chemistry climate models. J. Geophys. Res. 118, 73–84 (2013)
Olsen, M. A., Schoeberl, M. R. & Nielsen, J. E. Response of stratospheric circulation and stratosphere-troposphere exchange to changing sea surface temperatures. J. Geophys. Res. 112, (2007)
Sherwen, T., Evans, M. J., Carpenter, L. J., Schmidt, J. A. & Mickley, L. J. Halogen chemistry reduces tropospheric O3 radiative forcing. Atmos. Chem. Phys. 17, 1557–1569 (2017)
Sprenger, M., Wernli, H. & Bourqui, M. Stratosphere–troposphere exchange and its relation to potential vorticity streamers and cutoffs near the extratropical tropopause. J. Atmos. Sci. 64, 1587–1602 (2007)
Xie, B., Zhang, H., Wang, Z., Zhao, S. & Fu, Q. A modeling study of effective radiative forcing and climate response due to tropospheric ozone. Adv. Atmos. Sci. 33, 819–828 (2016)
Knutti, R., Fluckiger, J., Stocker, T. F. & Timmermann, A. Strong hemispheric coupling of glacial climate through freshwater discharge and ocean circulation. Nature 430, 851–856 (2004)
McManus, J. F., Francois, R., Gherardi, J. M., Keigwin, L. D. & Brown-Leger, S. Collapse and rapid resumption of Atlantic meridional circulation linked to deglacial climate changes. Nature 428, 834–837 (2004)
Persechino, A. et al. Decadal-timescale changes of the Atlantic overturning circulation and climate in a coupled climate model with a hybrid-coordinate ocean component. Clim. Dyn. 39, 1021–1042 (2012)
Shepherd, T. G. & McLandress, C. A robust mechanism for strengthening of the Brewer–Dobson circulation in response to climate change: critical-layer control of subtropical wave breaking. J. Atmos. Sci. 68, 784–797 (2011)
Miller, G. H. et al. Arctic amplification: can the past constrain the future? Quat. Sci. Rev. 29, 1779–1790 (2010)
Butchart, N. et al. Simulations of anthropogenic change in the strength of the Brewer-Dobson circulation. Clim. Dyn. 27, 727–741 (2006)
Holmes, C. D., Prather, M. J., Sovde, O. A. & Myhre, G. Future methane, hydroxyl, and their uncertainties: key climate and emission parameters for future predictions. Atmos. Chem. Phys. 13, 285–302 (2013)
Allan, W., Struthers, H. & Lowe, D. C. Methane carbon isotope effects caused by atomic chlorine in the marine boundary layer: global model results compared with Southern Hemisphere measurements. J. Geophys. Res. 112, D04306 (2007)
Brook, E. J., Sowers, T. & Orchardo, J. Rapid variations in atmospheric methane concentration during the past 110,000 years. Science 273, 1087–1091 (1996)
Grootes, P. M. & Stuiver, M. Oxygen 18/16 variability in Greenland snow and ice with 10−3- to 105-year time resolution. J. Geophys. Res. 102, 26455–26470 (1997)
CLIMAP. The surface of the ice-age Earth. Science 191, 1131–1137 (1976)
Webb, R. S., Rind, D. H., Lehman, S. J., Healy, R. J. & Sigman, D. Influence of ocean heat transport on the climate of the Last Glacial Maximum. Nature 385, 695–699 (1997)
Cuffey, K. M. & Clow, G. D. Temperature, accumulation, and ice sheet elevation in central Greenland through the last deglacial transition. J. Geophys. Res. 102, 26383–26396 (1997)
Geng, L. et al. Analysis of oxygen-17 excess of nitrate and sulfate at sub-micromole levels using the pyrolysis method. Rapid Commun. Mass Spectrom. 27, 2411–2419 (2013)
Geng, L. et al. On the origin of the occasional spring nitrate peak in Greenland snow. Atmos. Chem. Phys. 14, 13361–13376 (2014)
Kaiser, J., Hastings, M. G., Houlton, B. Z., Rockmann, T. & Sigman, D. M. Triple oxygen isotope analysis of nitrate using the denitrifier method and thermal decomposition of N2O. Anal. Chem. 79, 599–607 (2007)
Gupta, P., Noone, D., Galewsky, J., Sweeney, C. & Vaughn, B. H. Demonstration of high-precision continuous measurements of water vapor isotopologues in laboratory and remote field deployments using wavelength-scanned cavity ring-down spectroscopy (WS-CRDS) technology. Rapid Commun. Mass Spectrom. 23, 2534–2542 (2009)
Kaplan, J. O., Folberth, G. & Hauglustaine, D. A. Role of methane and biogenic volatile organic compound sources in late glacial and Holocene fluctuations of atmospheric methane concentrations. Glob. Biogeochem. Cycles 20, GB2016 (2006)
Pfeiffer, M., Spessa, A. & Kaplan, J. O. A model for global biomass burning in preindustrial time: LPJ-LMfire (v1.0). Geosci. Model Dev. 6, 643–685 (2013)
Alexander, M. J. et al. Recent developments in gravity-wave effects in climate models and the global distribution of gravity-wave momentum flux from observations and models. Q. J. R. Meteorol. Soc. 136, 1103–1124 (2010)
Acknowledgements
We acknowledge financial support from NSF awards AGS 1103163, PLR 1106317 and PLR 1244817 (to B.A.) and AGS 1102880 (to L.J.M. and L.T.M.). L.T.M. was also supported by the NASA Postdoctoral Program Fellowship administered by Oak Ridge Associated Universities (NNH06CC03B). Q.F. is supported by NASA Grant NNX13AN49G. P.L. is supported by NA14OAR4320106 from the National Oceanic and Atmospheric Administration, the US Department of Commerce. The statements, findings, conclusions, and recommendations are those of the author(s) and do not necessarily reflect the views of the National Oceanic and Atmospheric Administration, or the US Department of Commerce. We thank the National Ice Core Laboratory for providing the GISP2 ice-core samples, and the GISP2 team for ice-core drilling. We also thank our laboratory technician B. Vanden Heuvel for measurements of δ18O(H2O).
Author information
Authors and Affiliations
Contributions
B.A. conceived the study; L.G. performed the measurements, analysed the experimental and model data, proposed the hypotheses and wrote the manuscript with B.A.; L.T.M. constructed the ICECAP model under the supervision of L.J.M., and provided the model results; L.T.M., P.L. and Q.F. contributed to the hypotheses; A.J.S. assisted with the laboratory work. All authors contributed to the data interpretation and writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks T. Roeckmann and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Supplementary information
Supplementary Information
This file contains a Supplementary Discussion, Supplementary Table 1 and Supplementary References. (PDF 908 kb)
Rights and permissions
About this article
Cite this article
Geng, L., Murray, L., Mickley, L. et al. Isotopic evidence of multiple controls on atmospheric oxidants over climate transitions. Nature 546, 133–136 (2017). https://doi.org/10.1038/nature22340
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature22340
This article is cited by
-
Reactive aldehyde chemistry explains the missing source of hydroxyl radicals
Nature Communications (2024)
-
Changes in atmospheric oxidants over Arctic Ocean atmosphere: evidence of oxygen isotope anomaly in nitrate aerosols
npj Climate and Atmospheric Science (2023)
-
The self-cleansing ability of prehistoric air
Nature (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.