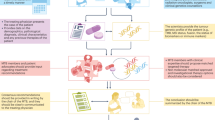
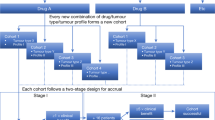
Abstract
An enhanced understanding of the molecular pathology of disease gained from genomic studies is facilitating the development of treatments that target discrete molecular subclasses of tumours. Considerable associated challenges include how to advance and implement targeted drug-development strategies. Precision medicine centres on delivering the most appropriate therapy to a patient on the basis of clinical and molecular features of their disease. The development of therapeutic agents that target molecular mechanisms is driving innovation in clinical-trial strategies. Although progress has been made, modifications to existing core paradigms in oncology drug development will be required to realize fully the promise of precision medicine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Chin, L. & Gray, J. W. Translating insights from the cancer genome into clinical practice. Nature 452, 553–563 (2008). A review that outlines the opportunities, challenges and approaches associated with the advancement of genomics-based medicine.
Stratton, M. R. Exploring the genomes of cancer cells: progress and promise. Science 331, 1553–1558 (2011).
Stratton, M. R., Campbell, P. J. & Futreal, P. A. The cancer genome. Nature 458, 719–724 (2009). A review of recent progress in cancer genomics and the potential of its application to medicine.
Hudson, T. J. et al. International network of cancer genome projects. Nature 464, 993–998 (2010); erratum 465, 966 (2010).
The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455, 1061–1068 (2008); erratum 494, 506 (2013).
Chin, L., Andersen, J. N. & Futreal, P. A. Cancer genomics: from discovery science to personalized medicine. Nature Med. 17, 297–303 (2011). A review that addresses the accumulating knowledge acquired through large-scale genomic sequencing efforts and discusses strategies for translating these discoveries into patient care.
Zhang, B. et al. Proteogenomic characterization of human colon and rectal cancer. Nature 513, 382–387 (2014).
Verweij, J. et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 364, 1127–1134 (2004).
Gerber, D. E. & Minna, J. D. ALK inhibition for non-small cell lung cancer: from discovery to therapy in record time. Cancer Cell 18, 548–551 (2010).
Sosman, J. A. et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N. Engl. J. Med. 366, 707–714 (2012).
Slamon, D. et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 365, 1273–1283 (2011).
Shaw, A. T. et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 368, 2385–2394 (2013).
Maemondo, M. et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 362, 2380–2388 (2010).
Ledermann, J. et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 15, 852–861 (2014).
Kris, M. G. et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. J. Am. Med. Assoc. 311, 1998–2006 (2014).
Jänne, P. A. et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N. Engl. J. Med. 372, 1689–1699 (2015).
Demetri, G. D. et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 347, 472–480 (2002).
Green, E. D., Guyer, M. S. & National Human Genome Research Institute. Charting a course for genomic medicine from base pairs to bedside. Nature 470, 204–213 (2011). A Perspective article that describes the past, present and future trajectories of genomic medicine.
Von Hoff, D. D. et al. Pilot study using molecular profiling of patients' tumors to find potential targets and select treatments for their refractory cancers. J. Clin. Oncol. 28, 4877–4883 (2010). This paper and refs 20 and 21 are some of the first descriptions of the use of molecular targeted therapies to improve patient outcomes.
Tsimberidou, A.-M. et al. Personalized medicine in a phase I clinical trials program: the MD Anderson Cancer Center initiative. Clin. Cancer Res. 18, 6373–6383 (2012).
Hollebecque, A. et al. Molecular screening for cancer treatment optimization (MOSCATO 01): a prospective molecular triage trial — interim results. J. Clin. Oncol. 31, 2512 (2013).
Dienstmann, R. et al. Molecular profiling of patients with colorectal cancer and matched targeted therapy in phase I clinical trials. Mol. Cancer Ther. 11, 2062–2071 (2012).
Association of the British Pharmaceutical Industry. The Stratification of Disease for Personalised Medicines http://www.abpi.org.uk/our-work/library/medical-disease/Documents/strat_med.pdf (2014).
The Academy of Medical Sciences. Realising the Potential of Stratified Medicine https://www.acmedsci.ac.uk/viewFile/51e915f9f09fb.pdf (2013).
Cook, D. et al. Lessons learned from the fate of AstraZeneca's drug pipeline: a five-dimensional framework. Nature Rev. Drug Discov. 13, 419–431 (2014).
Lee, C. K., Lord, S. J., Coates, A. S. & Simes, R. J. Molecular biomarkers to individualise treatment: assessing the evidence. Med. J. Aust. 190, 631–636 (2009).
Sargent, D. J., Conley, B. A., Allegra, C. & Collette, L. Clinical trial designs for predictive marker validation in cancer treatment trials. J. Clin. Oncol. 23, 2020–2027 (2005). A description of the fundamental basis of clinical-trial designs that are used to assess biomarkers.
Mandrekar, S. J. & Sargent, D. J. Clinical trial designs for predictive biomarker validation: theoretical considerations and practical challenges. J. Clin. Oncol. 27, 4027–4034 (2009).
Printz, C. Failure rate: why many cancer drugs don't receive FDA approval, and what can be done about it. Cancer 121, 1529–1530 (2015).
Sleijfer, S., Bogaerts, J. & Siu, L. L. Designing transformative clinical trials in the cancer genome era. J. Clin. Oncol. 31, 1834–1841 (2013).
Kim, E. S. et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov. 1, 44–53 (2011).
Esserman, L. J. et al. Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res. Treat. 132, 1049–1062 (2012).
Esserman, L. J. et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL–CALGB 150007/150012, ACRIN 6657. J. Clin. Oncol. 30, 3242–3249 (2012).
Hylton, N. M. et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy–results from ACRIN 6657/I-SPY TRIAL. Radiology 263, 663–672 (2012).
Lin, C. et al. Locally advanced breast cancers are more likely to present as Interval Cancers: results from the I-SPY 1 TRIAL (CALGB 150007/150012, ACRIN 6657, InterSPORE Trial). Breast Cancer Res. Treat. 132, 871–879 (2012).
Lindsay, C. R., Shaw, E., Walker, I. & Johnson, P. W. Lessons for molecular diagnostics in oncology from the Cancer Research UK Stratified Medicine Programme. Expert Rev. Mol. Diagn. 15, 287–289 (2015).
Le, D. T. et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Le Tourneau, C. et al. Designs and challenges for personalized medicine studies in oncology: focus on the SHIVA trial. Target. Oncol. 7, 253–265 (2012).
Le Tourneau, C. et al. Randomized phase II trial comparing molecularly targeted therapy based on tumor molecular profiling versus conventional therapy in patients with refractory cancer: results of the SHIVA trial. J. Clin. Oncol. 33, 11113 (2015).
Watson, I. R., Takahashi, K., Futreal, P. A. & Chin, L. Emerging patterns of somatic mutations in cancer. Nature Rev. Genet. 14, 703–718 (2013).
US Food and Drug Administration. Nucleic Acid Based Tests. US Food and Drug Administration http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm330711.htm (2015).
US Food and Drug Administration. List of Cleared or Approved Companion Diagnostic Devices (In vitro and Imaging Tools). US Food and Drug Administration http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm301431.htm (2015).
US Food and Drug Administration. Drug Approvals and Databases. US Food and Drug Administration http://www.fda.gov/Drugs/InformationOnDrugs/ (2015).
European Medicines Agency. European public assessment reports. European Medicines Agency http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp&mid=WC0b01ac058001d125 (2015).
Hay, M., Thomas, D. W., Craighead, J. L., Economides, C. & Rosenthal, J. Clinical development success rates for investigational drugs. Nature Biotechnol. 32, 40–51 (2014).
Yap, T. A., Sandhu, S. K., Workman, P. & de Bono, J. S. Envisioning the future of early anticancer drug development. Nature Rev. Cancer 10, 514–523 (2010).
Chantrill, L. A. et al. Precision medicine for advanced pancreas cancer: the Individualized Molecular Pancreatic Cancer Therapy (IMPaCT) trial. Clin. Cancer Res. 21, 2029–2037 (2015).
National Cancer Institute Molecular Analysis for Therapy Choice http://deainfo.nci.nih.gov/advisory/ncab/164_1213/Conley.pdf.
Lung Cancer Master Protocol (Lung-MAP) Clinical Trials. About Lung-MAP. Lung-MAP http://www.lung-map.org/about-lung-map (2015).
EORTC. About SPECTAColor. SPECTAColor EORTC Colorectal Cancer Screening Platform http://spectacolor.eortc.org/about (2015).
EORTC. EORTC, through SPECTAlung, participates in EU consortium validating blood-based cancer biomarkers. EORTC The future of cancer therapy http://www.eortc.org/news/eortc-through-spectalung-participates-in-european-consortium-validating-blood-based-cancer-biomarkers/ (2015).
Zardavas, D. et al. The AURORA initiative for metastatic breast cancer. Br. J. Cancer 111, 1881–1887 (2014).
Matsumoto, S. et al. Nationwide genomic screening network for the development of novel targeted therapies in advanced non-small cell lung cancer (LC-SCRUM-Japan). J. Clin. Oncol. 33 (suppl. 15), 8093 (2015).
Kalf, R. R. et al. Variations in predicted risks in personal genome testing for common complex diseases. Genet. Med. 16, 85–91 (2014).
Roper, N., Stensland, K. D., Hendricks, R. & Galsky, M. D. The landscape of precision cancer medicine clinical trials in the United States. Cancer Treat. Rev. 41, 385–390 (2015).
Simon, R. & Roychowdhury, S. Implementing personalized cancer genomics in clinical trials. Nature Rev. Drug Discov. 12, 358–369 (2013).
Waddell, N. et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 518, 495–501 (2015). A report that demonstrates how different genomic readouts could be important biomarkers for therapeutic responsiveness.
Alexandrov, L. B., Nik-Zainal, S., Wedge, D. C., Campbell, P. J. & Stratton, M. R. Deciphering signatures of mutational processes operative in human cancer. Cell Rep. 3, 246–259 (2013).
Frampton, G. M. et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nature Biotechnol. 31, 1023–1031 (2013).
Dawson, S. J. et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N. Engl. J. Med. 368, 1199–1209 (2013).
Douillard, J. Y. et al. Gefitinib treatment in EGFR mutated caucasian NSCLC: circulating-free tumor DNA as a surrogate for determination of EGFR status. J. Thorac. Oncol. 9, 1345–1353 (2014).
Lewin, J. & Siu, L. L. Cancer genomics: the challenge of drug accessibility. Curr. Opin. Oncol. 27, 250–257 (2015).
Lara, P. N. Jr et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J. Clin. Oncol. 19, 1728–1733 (2001).
Institute of Medicine. Transforming Clinical Research in the United States: Challenges and Opportunities: Workshop Summary (The National Academies Press, 2010). Part of a report from a workshop at which issues relating to clinical-trial-recruitment statistics were presented and specific challenges were identified.
Cancer Research UK. Stratified medicine and the lung cancer 'Matrix' trial — part of a cancer care revolution. Cancer Research UK http://scienceblog.cancerresearchuk.org/2014/04/17/stratified-medicine-and-the-lung-cancer-matrix-trial-part-of-a-cancer-care-revolution (2014).
Tam, A. L. et al. Feasibility of image-guided transthoracic core-needle biopsy in the BATTLE lung trial. J. Thorac. Oncol. 8, 436–442 (2013).
Seguin, L. et al. An integrin β3–KRAS–RalB complex drives tumour stemness and resistance to EGFR inhibition. Nature Cell Biology. 16, 457–468 (2014).
ISPY-2 Clinical Trials. About. I-SPY 2 TRIAL http://ispy2.org/about (2015).
Barker, A. D. et al. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin. Pharmacol. Ther. 86, 97–100 (2009).
National Institutes of Health. Molecular profiling-based assignment of cancer therapy for patients with advanced solid tumors. National Institutes of Health Clinical Center http://clinicalstudies.info.nih.gov/cgi/detail.cgi?A_2013-C-0105.html.
TrialReach. Clinical study for patients with cancer (Ve-Basket 120326). TrialReach http://trialreach.com/study/clinical-study-for-patients-with-cancer-ve-basket-/CT120326/ (2012).
Worldwide International Networking. WIN Clinical Trials/Scientific Projects. Worldwide International Networking in personalised cancer medicine http://www.winconsortium.org/page.jsp?id=104.
André, F. et al. Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: a multicentre, prospective trial (SAFIR01/UNICANCER). Lancet Oncol. 15, 267–274 (2014).
Medical Research Council Clinical Trials Unit. Welcome to FOCUS4. FOCUS4 Molecular selection of therapy in metastatic colorectal cancer: a molecularly stratified randomised controlled trial programme http://www.focus4trial.org/ (2014).
Biankin, A. V. & Hudson, T. J. Somatic variation and cancer: therapies lost in the mix. Hum. Genet. 130, 79–91 (2011). A review article that addresses the challenges presented by the molecular diversity in cancer that is uncovered through genomic sequencing.
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Acknowledgements
The authors would like to thank A. Ewing for her assistance in compiling the manuscript. They also thank L. Musgrove, D. Chang and P. Bailey for proofreading the manuscript and for their helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reprints and permissions information is available at www.nature.com/reprints.
Rights and permissions
About this article
Cite this article
Biankin, A., Piantadosi, S. & Hollingsworth, S. Patient-centric trials for therapeutic development in precision oncology. Nature 526, 361–370 (2015). https://doi.org/10.1038/nature15819
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature15819
This article is cited by
-
Glycerol-weighted chemical exchange saturation transfer nanoprobes allow 19F/1H dual-modality magnetic resonance imaging-guided cancer radiotherapy
Nature Communications (2023)
-
Modelling acute myeloid leukemia (AML): What’s new? A transition from the classical to the modern
Drug Delivery and Translational Research (2023)
-
First-line pyrotinib in advanced HER2-mutant non-small-cell lung cancer: a patient-centric phase 2 trial
Nature Medicine (2023)
-
Predictive Value of EGFR-PI3K-AKT-mTOR-Pathway Inhibitor Biomarkers for Head and Neck Squamous Cell Carcinoma: A Systematic Review
Molecular Diagnosis & Therapy (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.