Abstract
To contend with hazards posed by environmental fluoride, microorganisms export this anion through F−-specific ion channels of the Fluc family1,2,3,4. Since the recent discovery of Fluc channels, numerous idiosyncratic features of these proteins have been unearthed, including strong selectivity for F− over Cl− and dual-topology dimeric assembly5,6. To understand the chemical basis for F− permeation and how the antiparallel subunits convene to form a F−-selective pore, here we solve the crystal structures of two bacterial Fluc homologues in complex with three different monobody inhibitors, with and without F− present, to a maximum resolution of 2.1 Å. The structures reveal a surprising ‘double-barrelled’ channel architecture in which two F− ion pathways span the membrane, and the dual-topology arrangement includes a centrally coordinated cation, most likely Na+. F− selectivity is proposed to arise from the very narrow pores and an unusual anion coordination that exploits the quadrupolar edges of conserved phenylalanine rings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Baker, J. L. et al. Widespread genetic switches and toxicity resistance proteins for fluoride. Science 335, 233–235 (2012)
Li, S. et al. Eukaryotic resistance to fluoride toxicity mediated by a widespread family of fluoride export proteins. Proc. Natl Acad. Sci. USA 110, 19018–19023 (2013)
Ji, C., Stockbridge, R. B. & Miller, C. Bacterial fluoride resistance, Fluc channels, and the weak acid accumulation effect. J. Gen. Physiol. 144, 257–261 (2014)
Smith, K. D. et al. Yeast FEX1 is a constitutively expressived fluoride channel in with functional asymmetry of its two homologous domains. J. Biol. Chem. 290, 19874–19887 (2015)
Stockbridge, R. B., Robertson, J. L., Kolmakova-Partensky, L. & Miller, C. A family of fluoride-specific ion channels with dual-topology architecture. eLife 2, e01084. (2013)
Stockbridge, R. B., Koide, A., Miller, C. & Koide, S. Proof of dual-topology architecture of Fluc F− channels with monobody blockers. Nature Commun. 5, 5120 (2014)
Adamek, E., Pawlowska-Goral, K. & Bober, K. In vitro and in vivo effects of fluoride ions on enzyme activity. Ann. Acad. Med. Stetin. 51, 69–85 (2005)
Stockbridge, R. B. et al. Fluoride resistance and transport by riboswitch-controlled CLC antiporters. Proc. Natl Acad. Sci. USA 109, 15289–15294 (2012)
Ubarretxena-Belandia, I., Baldwin, J. M., Schuldiner, S. & Tate, C. G. Three-dimensional structure of the bacterial multidrug transporter EmrE shows it is an asymmetric homodimer. EMBO J. 22, 6175–6181 (2003)
Schuldiner, S. EmrE, a model for studying evolution and mechanism of ion-coupled transporters. Biochim. Biophys. Acta 1794, 748–762 (2009)
Morrison, E. A. et al. Antiparallel EmrE exports drugs by exchanging between asymmetric structures. Nature 481, 45–50 (2011)
Miller, C. Open-state substructure of single chloride channels from Torpedo electroplax. Philos. Trans. R. Soc. Lond. B 299, 401–411 (1982)
Rapp, M., Granseth, E., Seppala, S. & von Heijne, G. Identification and evolution of dual-topology membrane proteins. Nature Struct. Mol. Biol. 13, 112–116 (2006)
Koide, A., Wojcik, J., Gilbreth, R. N., Hoey, R. J. & Koide, S. Teaching an old scaffold new tricks: monobodies constructed using alternative surfaces of the FN3 scaffold. J. Mol. Biol. 415, 393–405 (2012)
Turman, D. L., Nathanson, J. T., Stockbridge, R. B., Street, T. O. & Miller, C. Two-sided block of a dual-topology F– channel. Proc. Natl Acad. Sci. USA 112, 5697–5701 (2015)
Harding, M. M. Metal-ligand geometry relevant to proteins and in proteins: sodium and potassium. Acta Crystallogr. D 58, 872–874 (2002)
Varma, S., Sabo, D. & Rempe, S. B. K+/Na+ selectivity in K channels and valinomycin: over-coordination versus cavity-size constraints. J. Mol. Biol. 376, 13–22 (2008)
Lev, B. R. B. & Noskov, S. Y. in Encyclopedia of Metalloproteins (eds Kretsinger, R. H. et al.) 2112–2118 (Springer, 2013)
Philip, V. et al. A survey of aspartate-phenylalanine and glutamate-phenylalanine interactions in the protein data bank: searching for anion-π pairs. Biochemistry 50, 2939–2950 (2011)
Allen, F. H. The Cambridge Structural Database: a quarter of a million crystal structures and rising. Acta Crystallogr. B 58, 380–388 (2002)
Kane Dickson, V., Pedi, L. & Long, S. B. Structure and insights into the function of a Ca2+-activated Cl− channel. Nature 516, 213–218 (2014)
Walden, M. et al. Uncoupling and turnover in a Cl−/H+ exchange transporter. J. Gen. Physiol. 129, 317–329 (2007)
Brammer, A. E., Stockbridge, R. B. & Miller, C. F. F−/Cl− selectivity in CLCF-type F−/H+ antiporters. J. Gen. Physiol. 144, 129–136 (2014)
Weinreich, F. & Jentsch, T. J. Pores formed by single subunits in mixed dimers of different CLC chloride channels. J. Biol. Chem. 276, 2347–2353 (2001)
Hille, B. & Schwarz, W. Potassium channels as multi-ion single-file pores. J. Gen. Physiol. 72, 409–442 (1978)
Morais-Cabral, J. H., Zhou, Y. & MacKinnon, R. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature 414, 37–42 (2001)
Caffrey, M. & Cherezov, V. Crystallizing membrane proteins using lipidic mesophases. Nature Protocols 4, 706–731 (2009)
Winter, G., Lobley, C. M. & Prince, S. M. Decision making in xia2. Acta Crystallogr. D 69, 1260–1273 (2013)
Kabsch, W. Xds. Acta Crystallogr. D 66, 125–132 (2010)
Evans, P. R. An introduction to data reduction: space-group determination, scaling and intensity statistics. Acta Crystallogr. D 67, 282–292 (2011)
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008)
Bricogne, G. et al. Generation, representation and flow of phase information in structure determination: recent developments in and around SHARP 2.0. Acta Crystallogr. D 59, 2023–2030 (2003)
Abrahams, J. P. & Leslie, A. G. Methods used in the structure determination of bovine mitochondrial F1 ATPase. Acta Crystallogr. D 52, 30–42 (1996)
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007)
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991)
Vagin, A. & Teplyakov, A. Molecular replacement with MOLREP. Acta Crystallogr. D 66, 22–25 (2010)
Murshudov, G. N. et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D 67, 355–367 (2011)
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D 67, 235–242 (2011)
Chen, V. B. et al. Molprobity: all-atom structure validataion for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010)
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010)
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66 486–501 http://dx.doi.org/10.1107/S0907444910007493 (2010)
Acknowledgements
We are grateful to J. Parker for critically reading the manuscript; to D. Turman, A. Lajoie, M. Pham, and K. Piasta for pilot experiments; to S. Strobel for sharing results of unpublished experiments; and to B. Foxman for help with the Cambridge Structural Database. This work was supported in part by a Wellcome Trust Investigator Award 102890/Z/13/Z and National Institutes of Health (NIH) grants RO1-GM107023 and U54-GM087519. R.B.S. was supported by NIH grant K99-GM-111767. C.M. is grateful to F. Ashcroft and Lincoln College, Oxford, for hosting a Newton-Abraham Visiting Professorship.
Author information
Authors and Affiliations
Contributions
R.B.S., S.N. and C.M. performed experiments, analysed the data and wrote the paper. L.K.-P. and T.S. performed experiments. S.K. and A.K. provided monobody clones and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Crystal lattices for the Bpe–S7, Bpe–L2 and Ec2–S9 crystal structures.
The asymmetric unit is shown in green and red (channel and monobody, respectively), and symmetry mates are shown in black and blue.
Extended Data Figure 2 Bpe–L2 complex.
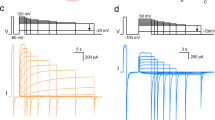
Left, cartoon schematic of Bpe crystal structure, coloured as in Fig. 1b. The variable regions of monobody L2 are coloured cyan. Mesh-rendering is shown for the lower monobody. Right, single-channel recording of Bpe in the presence of 200 nM L2. Zero-current level is indicated by the dashed line.
Extended Data Figure 3 Stereo images of Bpe–L2.
a–d, Stereo images corresponding to the structures shown in Fig. 2a–d.
Extended Data Figure 4 Single channel trace of Bpe in Na+-free recording solution, with addition of 200 nM blocking monobody L3.
Channels were recorded in the presence of 300 mM N-methyl-glucamine-fluoride, from which all small cations were rigorously excluded. The zero-current level is indicated by the dashed line.
Extended Data Figure 5 Experimental electron density for the Ec2–S9 crystal structure.
Left, cartoon schematic of Ec2 with S9 monobodies bound, coloured as in Bpe in Fig. 1b. Variable sequences of the monobodies (cyan) with ribbon or mesh representation. Right, cartoon view of TM4 from Ec2, with the solvent-flattened electron density map calculated from SHARP contoured at 1.8σ (blue), and anomalous difference density from seleno-l-methionine contoured at 5σ (magenta).
Extended Data Figure 6 Liposome flux assays of Bpe variants.
Top three panels: F− transport from liposomes by Bpe mutants F82I, F85I, and N43D, in the presence and absence of 6 μM blocking monobody. F− efflux from proteoliposomes (0.2 μg protein per mg lipid for Phe mutants; 1 μg protein per mg lipid for N43D) was monitored with a F− electrode and normalized against total trapped F−. Bottom panel: F− dump by F85I measured at pH 7 and pH 9. Rates are summarized in Extended Data Table 2.
Extended Data Figure 7 Sequence alignment of eukaryotic N- and C-terminal Fluc domain sequences, with bacterial homodimer sequences below.
Highly conserved residues are shaded in grey. For the eukaryotic sequences, residues expected to line ‘pore 2’, the pore mostly encompassed by the C-terminal domain, are coloured red.
Rights and permissions
About this article
Cite this article
Stockbridge, R., Kolmakova-Partensky, L., Shane, T. et al. Crystal structures of a double-barrelled fluoride ion channel. Nature 525, 548–551 (2015). https://doi.org/10.1038/nature14981
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14981
This article is cited by
-
The structural basis of promiscuity in small multidrug resistance transporters
Nature Communications (2020)
-
Fast and selective fluoride ion conduction in sub-1-nanometer metal-organic framework channels
Nature Communications (2019)
-
Succinate-acetate permease from Citrobacter koseri is an anion channel that unidirectionally translocates acetate
Cell Research (2018)
-
A mighty stream of membrane proteins
Nature Structural & Molecular Biology (2018)
-
Crystal structure of undecaprenyl-pyrophosphate phosphatase and its role in peptidoglycan biosynthesis
Nature Communications (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.