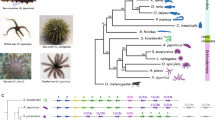
Abstract
Our understanding of vertebrate origins is powerfully informed by comparative morphology, embryology and genomics of chordates, hemichordates and echinoderms, which together make up the deuterostome clade. Striking body-plan differences among these phyla have historically hindered the identification of ancestral morphological features, but recent progress in molecular genetics and embryology has revealed deep similarities in body-axis formation and organization across deuterostomes, at stages before morphological differences develop. These developmental genetic features, along with robust support of pharyngeal gill slits as a shared deuterostome character, provide the foundation for the emergence of chordates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bateson, W. The ancestry of the chordata. Q. J. Microsc. Sci. 26, 535–571 (1886).
Kowalevsky, A. Anatomie des Balanoglossus [in French]. Mem. Acad. Imp. Sci. St Petersb. 7, 16 (1866).
Metchnikoff, V. E. Über die systematische Stellung von Balanoglossus [in German]. Zool. Anz. 4, 139–157 (1881).
Carroll, S. B., Grenier, J. K. & Weatherbee, S. D. From DNA to Diversity: Molecular Genetics and the Evolution of Animal Design 2nd edn (Blackwell, 2005).
De Robertis, E. M. Evo-devo: variations on ancestral themes. Cell 132, 185–195 (2008).
Brown, F. D., Prendergast, A. & Swalla, B. J. Man is but a worm: chordate origins. Genesis 46, 605–613 (2008).
Gerhart, J., Lowe, C. & Kirschner, M. Hemichordates and the origin of chordates. Curr. Opin. Genet. Dev. 15, 461–467 (2005).
Holland, L. Z. Chordate roots of the vertebrate nervous system: expanding the molecular toolkit. Nature Rev. Neurosci. 10, 736–746 (2009).
Lapraz, F., Besnardeau, L. & Lepage, T. Patterning of the dorsal-ventral axis in echinoderms: insights into the evolution of the BMP-chordin signaling network. PLoS Biol. 7, e1000248 (2009).
Satoh, N. Developmental Genomics of Ascidians (Wiley-Blackwell, 2014).
Yu, J. K. et al. Axial patterning in cephalochordates and the evolution of the organizer. Nature 445, 613–617 (2007). This article reports molecular evidence for a functional organizer in amphioxus, and supports the presence of this molecular patterning module at the base of the chordates.
Röttinger, E. & Lowe, C. J. Evolutionary crossroads in developmental biology: hemichordates. Development 139, 2463–2475 (2012).
Angerer, L. M., Yaguchi, S., Angerer, R. C. & Burke, R. D. The evolution of nervous system patterning: insights from sea urchin development. Development 138, 3613–3623 (2011).
Peter, I. S. & Davidson, E. H. A gene regulatory network controlling the embryonic specification of endoderm. Nature 474, 635–639 (2011).
Swalla, B. J. & Smith, A. B. Deciphering deuterostome phylogeny: molecular, morphological and palaeontological perspectives. Phil. Trans. R. Soc. Lond. B 363, 1557–1568 (2008).
Haeckel, E. H. P. A. Anthropogenie: oder, Entwickelungsgeschichte des Menschen [in German] (Wilhelm Engelmann, 1874).
Kowalevsky, A. Weitere Studien über die Entwicklung der einfachen Ascidien [in German]. Arch. Mikr. Anat. 7, 101–130 (1866).
Darwin, C. The Descent of Man, and Selection in Relation to Sex (D. Appleton and company, 1871).
Grobben, K. Die systematische einteilung des tierreiches [in German]. Verh. der Zool.-Bot. Ges. Wien. 58, 491–511 (1908).
Martin-Duran, J. M., Janssen, R., Wennberg, S., Budd, G. E. & Hejnol, A. Deuterostomic development in the protostome Priapulus caudatus. Curr. Biol. 22, 2161–2166 (2012).
Delsuc, F., Brinkmann, H., Chourrout, D. & Philippe, H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 439, 965–968 (2006). The authors of this paper provide conclusive molecular evidence for the sister relationship between tunicates and vertebrates and the basal position of cephalochordates.
Dunn, C. W. et al. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452, 745–749 (2008).
Wada, H. & Satoh, N. Details of the evolutionary history from invertebrates to vertebrates, as deduced from the sequences of 18S rDNA. Proc. Natl Acad. Sci. USA 91, 1801–1804 (1994).
Blair, J. E. & Hedges, S. B. Molecular phylogeny and divergence times of deuterostome animals. Mol. Biol. Evol. 22, 2275–2284 (2005).
Field, K. G. et al. Molecular phylogeny of the animal kingdom. Science 239, 748–753 (1988).
Halanych, K. M. The phylogenetic position of the pterobranch hemichordates based on 18S rDNA sequence data. Mol. Phylogenet. Evol. 4, 72–76 (1995).
Philippe, H. et al. Acoelomorph flatworms are deuterostomes related to Xenoturbella. Nature 470, 255–258 (2011).
Hejnol, A. et al. Assessing the root of bilaterian animals with scalable phylogenomic methods. Proc. Biol. Sci. 276, 4261–4270 (2009).
Gee, H. Before the Backbone (Chapman & Hall, 1996).
Stach, T. Chordate phylogeny and evolution: a not so simple three-taxon problem. J. Zool. 276, 117–141 (2008).
Lu, T. M., Luo, Y. J. & Yu, J. K. BMP and Delta/Notch signaling control the development of amphioxus epidermal sensory neurons: insights into the evolution of the peripheral sensory system. Development 139, 2020–2030 (2012).
Escriva, H., Holland, N. D., Gronemeyer, H., Laudet, V. & Holland, L. Z. The retinoic acid signaling pathway regulates anterior/posterior patterning in the nerve cord and pharynx of amphioxus, a chordate lacking neural crest. Development 129, 2905–2916 (2002).
Onai, T. et al. Retinoic acid and Wnt/β-catenin have complementary roles in anterior/posterior patterning embryos of the basal chordate amphioxus. Dev. Biol. 332, 223–233 (2009).
Meulemans, D. & Bronner-Fraser, M. Gene-regulatory interactions in neural crest evolution and development. Dev. Cell 7, 291–299 (2004).
Shimeld, S. M. The evolution of the hedgehog gene family in chordates: insights from amphioxus hedgehog. Dev. Genes Evol. 209, 40–47 (1999).
Holland, L. Z. et al. Evolution of bilaterian central nervous systems: a single origin? EvoDevo 4, 27 (2013).
Irimia, M. et al. Conserved developmental expression of Fezf in chordates and Drosophila and the origin of the Zona Limitans Intrathalamica (ZLI) brain organizer. EvoDevo 1, 7 (2010).
Abedin, M. & King, N. The premetazoan ancestry of cadherins. Science 319, 946–948 (2008).
Conklin, E. G. The embryology of amphioxus. J. Morphol. 54, 69–151 (1932).
Gomez, C. et al. Control of segment number in vertebrate embryos. Nature 454, 335–339 (2008).
Beaster-Jones, L. et al. Expression of somite segmentation genes in amphioxus: a clock without a wavefront? Dev. Genes Evol. 218, 599–611 (2008).
Bertrand, S. et al. Amphioxus FGF signaling predicts the acquisition of vertebrate morphological traits. Proc. Natl Acad. Sci. USA 108, 9160–9165 (2011).
Kozmik, Z. et al. Pax-Six-Eya-Dach network during amphioxus development: conservation in vitro but context specificity in vivo. Dev. Biol. 306, 143–159 (2007).
Mazet, F., Amemiya, C. T. & Shimeld, S. M. An ancient Fox gene cluster in bilaterian animals. Curr. Biol. 16, R314–316 (2006).
Jandzik, D. et al. Evolution of the new vertebrate head by co-option of an ancient chordate skeletal tissue. Nature 518, 534–537 (2015).
Rychel, A. L. & Swalla, B. J. Development and evolution of chordate cartilage. J. Exp. Zool. B Mol. Dev. Evol. 308, 325–335 (2007).
Wright, G. M., Keeley, F. W. & Robson, P. The unusual cartilaginous tissues of jawless craniates, cephalochordates and invertebrates. Cell Tissue Res. 304, 165–174 (2001).
Range, R. C., Angerer, R. C. & Angerer, L. M. Integration of canonical and noncanonical Wnt signaling pathways patterns the neuroectoderm along the anterior–posterior axis of sea urchin embryos. PLoS Biol. 11, e1001467 (2013).
Yankura, K. A., Martik, M. L., Jennings, C. K. & Hinman, V. F. Uncoupling of complex regulatory patterning during evolution of larval development in echinoderms. BMC Biol. 8, 143 (2010).
Sato, A., Bishop, J. D. & Holland, P. W. Developmental biology of pterobranch hemichordates: history and perspectives. Genesis 46, 587–591 (2008).
Dominguez, P., Jacobson, A. G. & Jefferies, R. P. Paired gill slits in a fossil with a calcite skeleton. Nature 417, 841–844 (2002).
Gonzalez, P. & Cameron, C. B. The gill slits and pre-oral ciliary organ of Protoglossus (Hemichordata: Enteropneusta) are filter-feeding structures. Biol. J. Linn. Soc. 98, 898–906 (2009).
Gillis, J. A., Fritzenwanker, J. H. & Lowe, C. J. A stem-deuterostome origin of the vertebrate pharyngeal transcriptional network. Proc. Biol. Sci. 279, 237–246 (2012). This report shows extensive patterning similarities between the development of chordate and enteropneust gills, further supporting morphological homology.
Ogasawara, M., Wada, H., Peters, H. & Satoh, N. Developmental expression of Pax1/9 genes in urochordate and hemichordate gills: insight into function and evolution of the pharyngeal epithelium. Development 126, 2539–2550 (1999).
Fritzenwanker, J. H., Gerhart, J., Freeman, R. M. Jr & Lowe, C. J. The Fox/Forkhead transcription factor family of the hemichordate Saccoglossus kowalevskii. EvoDevo 5, 17 (2014).
Balser, E. J. & Ruppert, E. E. Structure, ultrastructure, and function of the preoral heart-kidney in Saccoglossus kowalevskii (Hemichordata, Enteropneusta) including new data on the stomochord. Acta Zool. 71, 235–249 (1990).
Miyamoto, N. & Wada, H. Hemichordate neurulation and the origin of the neural tube. Nature Commun. 4, 2713 (2013). This manuscript demonstrates similar mediolateral patterning mechanisms between the hemichordate collar cord and chordate dorsal cord.
Hyman, L. H. The Invertebrates 1st edn (McGraw-Hill, 1959).
Luttrell, S., Konikoff, C., Byrne, A., Bengtsson, B. & Swalla, B. J. Ptychoderid hemichordate neurulation without a notochord. Integr. Comp. Biol. 52, 829–834 (2012).
Ruppert, E. E. Key characters uniting hemichordates and chordates: homologies or homoplasies? Can. J. Zool. 83, 8–23 (2005).
Satoh, N. et al. On a possible evolutionary link of the stomochord of hemichordates to pharyngeal organs of chordates. Genesis 52, 925–934 (2014).
De Robertis, E. M. & Kuroda, H. Dorso-ventral patterning and neural induction in Xenopus embryos. Annu. Rev. Cell Dev. Biol. 20, 285–308 (2004).
Pani, A. M. et al. Ancient deuterostome origins of vertebrate brain signalling centres. Nature 483, 289–294 (2012). This paper presents evidence that ectodermal signalling centres thought to have been uniquely associated with the evolution of vertebrate brains are present in hemichordates as part of a conserved ancient deuterostome patterning network.
Bullock, T. H. The anatomical organization of the nervous system of enteropneusta. Q. J. Microsc. Sci. 86, 55–111 (1945).
Kaul, S. & Stach, T. Ontogeny of the collar cord: neurulation in the hemichordate Saccoglossus kowalevskii. J. Morphol. 271, 1240–1259 (2010).
Knight-Jones, E. On the nervous system of Saccoglossus cambriensis (Enteropneusta). Phil. Trans. R. Soc. Lond. B 236, 315–354 (1952).
Morgan, T. Development of Balanoglossus. J. Morphol. 9, 1–86 (1894).
Benito-Gutierrez, E. & Arendt, D. CNS evolution: new insight from the mud. Curr. Biol. 19, R640–642 (2009).
Nomaksteinsky, M. et al. Centralization of the deuterostome nervous system predates chordates. Curr. Biol. 19, 1264–1269 (2009). This paper shows clear molecular evidence for the presence of cell bodies in the dorsal nerve cord of enteropneusts and proposes the deep ancestry of a CNS in the deuterostomes.
Bullock, T. H. The functional organisation of the nervous system of the Enteropneusta. Biol. Bull. 79, 91–113 (1940).
Cameron, C. B. & Mackie, G. O. Conduction pathways in the nervous system of Saccoglossus sp. (Enteropneusta). Can. J. Zool. 74, 15–19 (1996).
Lowe, C. J. et al. Dorsoventral patterning in hemichordates: insights into early chordate evolution. PLoS Biol. 4, e291 (2006). This manuscript demonstrates the role of BMP signalling in the formation of the DV axis of the enteropneust adult body plan.
Martindale, M. Q. & Hejnol, A. A developmental perspective: changes in the position of the blastopore during bilaterian evolution. Dev. Cell 17, 162–174 (2009).
Slack, J. M., Holland, P. W. & Graham, C. F. The zootype and the phylotypic stage. Nature 361, 490–492 (1993).
Darras, S., Gerhart, J., Terasaki, M., Kirschner, M. & Lowe, C. J. β-Catenin specifies the endomesoderm and defines the posterior organizer of the hemichordate Saccoglossus kowalevskii. Development 138, 959–970 (2011).
Wikramanayake, A. H., Huang, L. & Klein, W. H. β-Catenin is essential for patterning the maternally specified animal-vegetal axis in the sea urchin embryo. Proc. Natl Acad. Sci. USA 95, 9343–9348 (1998).
Henry, J. Q., Perry, K. J., Wever, J., Seaver, E. & Martindale, M. Q. β-Catenin is required for the establishment of vegetal embryonic fates in the nemertean, Cerebratulus lacteus. Dev. Biol. 317, 368–379 (2008).
Wikramanayake, A. H. et al. An ancient role for nuclear β-catenin in the evolution of axial polarity and germ layer segregation. Nature 426, 446–450 (2003).
Kimelman, D. Mesoderm induction: from caps to chips. Nature Rev. Genet. 7, 360–372 (2006).
Green, S. A., Norris, R. P., Terasaki, M. & Lowe, C. J. FGF signaling induces mesoderm in the hemichordate Saccoglossus kowalevskii. Development 140, 1024–1033 (2013).
Hinman, V. F. & Davidson, E. H. Evolutionary plasticity of developmental gene regulatory network architecture. Proc. Natl Acad. Sci. USA 104, 19404–19409 (2007).
Hikasa, H. & Sokol, S. Y. Wnt signaling in vertebrate axis specification. Cold Spring Harb. Perspect. Biol. 5, a007955 (2013).
Petersen, C. P. & Reddien, P. W. Wnt signaling and the polarity of the primary body axis. Cell 139, 1056–1068 (2009).
Kiecker, C. & Niehrs, C. A morphogen gradient of Wnt/β-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development 128, 4189–4201 (2001).
Lowe, C. J. et al. Anteroposterior patterning in hemichordates and the origins of the chordate nervous system. Cell 113, 853–865 (2003). This paper provides evidence for a conserved transcriptional gene regulatory network between hemichordates and chordates despite large organizational differences in their basic body plans.
Aronowicz, J. & Lowe, C. J. Hox gene expression in the hemichordate Saccoglossus kowalevskii and the evolution of deuterostome nervous systems. Integr. Comp. Biol. 46, 890–901 (2006).
Freeman, R. et al. Identical genomic organization of two hemichordate hox clusters. Curr. Biol. 22, 2053–2058 (2012).
Holland, L. Z. et al. Evolution of bilaterian central nervous systems: a single origin? EvoDevo 4, 27 (2013).
David, B. & Mooi, R. How Hox genes can shed light on the place of echinoderms among the deuterostomes. EvoDevo 5, 22 (2014).
Hara, Y. et al. Expression patterns of Hox genes in larvae of the sea lily Metacrinus rotundus. Dev. Genes Evol. 216, 797–809 (2006).
Morris, V. B. & Byrne, M. Oral-aboral identity displayed in the expression of HpHox3 and HpHox11/13 in the adult rudiment of the sea urchin Holopneustes purpurescens. Dev. Genes Evol. 224, 1–11 (2014).
Lacalli, T. Echinoderm conundrums: Hox genes, heterochrony, and an excess of mouths. Evodevo 5, 46 (2014).
Peterson, K. J., Arenas-Mena, C. & Davidson, E. H. The A/P axis in echinoderm ontogeny and evolution: evidence from fossils and molecules. Evol. Dev. 2, 93–101 (2000).
Omori, A., Akasaka, K., Kurokawa, D. & Amemiya, S. Gene expression analysis of Six3, Pax6, and Otx in the early development of the stalked crinoid Metacrinus rotundus. Gene Expr. Patterns 11, 48–56 (2011).
Arenas-Mena, C., Cameron, A. R. & Davidson, E. H. Spatial expression of Hox cluster genes in the ontogeny of a sea urchin. Development 127, 4631–4643 (2000).
De Robertis, E. M. & Sasai, Y. A common plan for dorsoventral patterning in Bilateria. Nature 380, 37–40 (1996).
Mizutani, C. M. & Bier, E. EvoD/Vo: the origins of BMP signalling in the neuroectoderm. Nature Rev. Genet. 9, 663–677 (2008).
Denes, A. S. et al. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in bilateria. Cell 129, 277–288 (2007).
Röttinger, E. & Martindale, M. Q. Ventralization of an indirect developing hemichordate by NiCl2 suggests a conserved mechanism of dorso-ventral (D/V) patterning in Ambulacraria (hemichordates and echinoderms). Dev. Biol. 354, 173–190 (2011).
Satoh, N. An aboral-dorsalization hypothesis for chordate origin. Genesis 46, 614–622 (2008).
Gerhart, J. Evolution of the organizer and the chordate body plan. Int. J. Dev. Biol. 45, 133–153 (2001).
Lauri, A. et al. Development of the annelid axochord: insights into notochord evolution. Science 345, 1365–1368 (2014).
Cameron, C. B., Garey, J. R. & Swalla, B. J. Evolution of the chordate body plan: new insights from phylogenetic analyses of deuterostome phyla. Proc. Natl Acad. Sci. USA 97, 4469–4474 (2000). This manuscript proposes that the extant enteropneust, rather than pterobranch, adult body plan may best represent ancestral deuterostome characters.
Erwin, D. H. et al. The Cambrian conundrum: early divergence and later ecological success in the early history of animals. Science 334, 1091–1097 (2011).
Erwin, D. H. & Valentine, J. The Cambrian Explosion: the Construction of Animal Biodiversity (Roberts and Company, 2013).
Peterson, K. J. & Butterfield, N. J. Origin of the Eumetazoa: testing ecological predictions of molecular clocks against the Proterozoic fossil record. Proc. Natl Acad. Sci. USA 102, 9547–9552 (2005).
Lacalli, T. C. The emergence of the chordate body plan: some puzzles and problems. Acta Zool. 91, 4–10 (2010).
Garstang, W. The morphology of the Tunicata. Q. J. Microsc. Sci. 72, 51–187 (1928).
Lacalli, T. C. Protochordate body plan and the evolutionary role of larvae: old controversies resolved? Can. J. Zool. 83, 216–224 (2005).
Sea Urchin Genome Sequencing Consortium. The genome of the sea urchin Strongylocentrotus purpuratus. Science 314, 941–952 (2006).
Freeman, R. M. Jr et al. cDNA sequences for transcription factors and signaling proteins of the hemichordate Saccoglossus kowalevskii: efficacy of the expressed sequence tag (EST) approach for evolutionary and developmental studies of a new organism. Biol. Bull. 214, 284–302 (2008).
Baughman, K. W. et al. Genomic organization of Hox and ParaHox clusters in the echinoderm, Acanthaster planci. Genesis 52, 952–958 (2014).
Delsuc, F., Brinkmann, H., Chourrout, D. & Philippe, H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 439, 965–968 (2006).
Nielsen, C. Origin of the chordate central nervous system — and the origin of chordates. Dev. Genes Evol. 209, 198–205 (1999).
Gudo, M. & Syed, T. 100 years of Deuterostomia (Grobben, 1908): Cladogenetic and anagenetic relations within the notoneuralia domain. http://arxiv.org/abs/0811.2189 (2008).
Jefferies, R. P. S. The Ancestry of the Vertebrates (Cambridge Univ. Press, 1986).
Swalla, B. J. Building divergent body plans with similar genetic pathways. Heredity 97, 235–243 (2006).
Bone, Q. The central nervous system in amphioxus. J. Comp. Neurol. 115, 27–51 (1960).
Wicht, H. & Lacalli, T. C. The nervous system of amphioxus: structure, development, and evolutionary significance. Can. J. Zool. 83, 122–150 (2005).
Cannon, J. T. et al. Phylogenomic resolution of the hemichordate and echinoderm clade. Current Biol. 24, 2827–2832 (2014).
Cameron, C. B. Particle retention and flow in the pharynx of the enteropneust worm Harrimania planktophilus: the filter-feeding pharynx may have evolved before the chordates. Biol. Bull. 202, 192–200 (2002).
Telford, M. J. et al. Phylogenomic analysis of echinoderm class relationships supports Asterozoa. Proc. Biol. Sci. 281, 20140479 (2014).
Acknowledgements
We thank K. Bertsche (http://wanderingfalcon.com), for the scientific illustrations, C. Patton and J. Watanabe for photography of invertebrate micrographs, K. Halanych and J. Cannon for providing pterobranch images, and J. Fritzenwanker for the German translation of Grobben, and helpful discussions. We apologize to authors whose work we were unable to cite due to space limitations, and thank M. Kirschner, A. Pani, T. Lacalli, N. Satoh and N. Holland for discussions that helped formulate these ideas. Support for this work was awarded to C.J.L from NASA (NNX13AI68G) and NSF (1258169), to D.M.M. from NSF (IOS1257040). D.S.R. is supported by the Okinawa Institute of Science and Technology and the US National Institutes of Heath through grant R01 GM086321. Work at the Joint Genome Institute is supported by the Office of Science of the US Department of Energy under contract number DE-AC02-05CH11231.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reprints and permissions information is available at www.nature.com/reprints.
Rights and permissions
About this article
Cite this article
Lowe, C., Clarke, D., Medeiros, D. et al. The deuterostome context of chordate origins. Nature 520, 456–465 (2015). https://doi.org/10.1038/nature14434
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature14434
This article is cited by
-
The Cambrian fossil Pikaia, and the origin of chordate somites
EvoDevo (2024)
-
The origins of gas exchange and ion regulation in fish gills: evidence from structure and function
Journal of Comparative Physiology B (2024)
-
The brain regulatory program predates central nervous system evolution
Scientific Reports (2023)
-
A radical evolutionary makeover gave echinoderms their unusual body plan
Nature (2023)
-
Ion regulation at gills precedes gas exchange and the origin of vertebrates
Nature (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.