Abstract
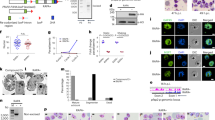
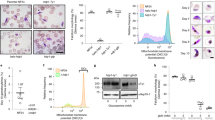
The life cycles of many parasites involve transitions between disparate host species, requiring these parasites to go through multiple developmental stages adapted to each of these specialized niches. Transmission of malaria parasites (Plasmodium spp.) from humans to the mosquito vector requires differentiation from asexual stages replicating within red blood cells into non-dividing male and female gametocytes. Although gametocytes were first described in 1880, our understanding of the molecular mechanisms involved in commitment to gametocyte formation is extremely limited, and disrupting this critical developmental transition remains a long-standing goal1. Here we show that expression levels of the DNA-binding protein PfAP2-G correlate strongly with levels of gametocyte formation. Using independent forward and reverse genetics approaches, we demonstrate that PfAP2-G function is essential for parasite sexual differentiation. By combining genome-wide PfAP2-G cognate motif occurrence with global transcriptional changes resulting from PfAP2-G ablation, we identify early gametocyte genes as probable targets of PfAP2-G and show that their regulation by PfAP2-G is critical for their wild-type level expression. In the asexual blood-stage parasites pfap2-g appears to be among a set of epigenetically silenced loci2,3 prone to spontaneous activation4. Stochastic activation presents a simple mechanism for a low baseline of gametocyte production. Overall, these findings identify PfAP2-G as a master regulator of sexual-stage development in malaria parasites and mark the first discovery of a transcriptional switch controlling a differentiation decision in protozoan parasites.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Accessions
Gene Expression Omnibus
Sequence Read Archive
Data deposits
Microarray data was submitted to the NCBI GEO repository (series accession GSE52030). Next generation sequencing data was submitted to the NCBI Sequence Read Archive (SRA) (study number ERP000190 for samples F12 (ERS011445), 3D7A (ERS011446) and GNP-A4 (ERS011447) and study number SRP035432 for samples E5 (SRS529791) and Δpfap2-g (SRS529811)).
References
Wells, T. N. C., Alonso, P. L. & Gutteridge, W. E. New medicines to improve control and contribute to the eradication of malaria. Nature Rev. Drug Discov. 8, 879–891 (2009)
Flueck, C. et al. Plasmodium falciparum heterochromatin protein 1 marks genomic loci linked to phenotypic variation of exported virulence factors. PLoS Pathog. 5, e1000569 (2009)
Lopez-Rubio, J. J., Mancio-Silva, L. & Scherf, A. Genome-wide analysis of heterochromatin associates clonally variant gene regulation with perinuclear repressive centers in malaria parasites. Cell Host Microbe 5, 179–190 (2009)
Cortés, A., Crowley, V. M., Vaquero, A. & Voss, T. S. A view on the role of epigenetics in the biology of malaria parasites. PLoS Pathog. 8, e1002943 (2012)
Alano, P. Plasmodium falciparum gametocytes: still many secrets of a hidden life. Mol. Microbiol. 66, 291–302 (2007)
Dixon, M. W. A., Thompson, J., Gardiner, D. L. & Trenholme, K. R. Sex in Plasmodium: a sign of commitment. Trends Parasitol. 24, 168–175 (2008)
Rovira-Graells, N. et al. Transcriptional variation in the malaria parasite Plasmodium falciparum. Genome Res. 22, 925–938 (2012)
Painter, H. J., Campbell, T. L. & Llinás, M. The Apicomplexan AP2 family: integral factors regulating Plasmodium development. Mol. Biochem. Parasitol. 176, 1–7 (2011)
Yuda, M. et al. Identification of a transcription factor in the mosquito-invasive stage of malaria parasites. Mol. Microbiol. 71, 1402–1414 (2009)
Yuda, M., Iwanaga, S., Shigenobu, S., Kato, T. & Kaneko, I. Transcription factor AP2-Sp and its target genes in malarial sporozoites. Mol. Microbiol. 75, 854–863 (2010)
Iwanaga, S., Kaneko, I., Kato, T. & Yuda, M. Identification of an AP2-family protein that is critical for malaria liver stage development. PLoS ONE 7, e47557 (2012)
Alano, P. et al. Plasmodium falciparum: parasites defective in early stages of gametocytogenesis. Exp. Parasitol. 81, 227–235 (1995)
Day, K. P. et al. Genes necessary for expression of a virulence determinant and for transmission of Plasmodium falciparum are located on a 0.3-megabase region of chromosome 9. Proc. Natl Acad. Sci. USA 90, 8292–8296 (1993)
Eksi, S. et al. Plasmodium falciparum gametocyte development 1 (Pfgdv1) and gametocytogenesis early gene identification and commitment to sexual development. PLoS Pathog. 8, e1002964 (2012)
Ikadai, H. et al. Transposon mutagenesis identifies genes essential for Plasmodium falciparum gametocytogenesis. Proc. Natl Acad. Sci. USA 110, E1676–E1684 (2013)
Manske, M. et al. Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature 487, 375–379 (2012)
Armstrong, C. M. & Goldberg, D. E. An FKBP destabilization domain modulates protein levels in Plasmodium falciparum. Nature Methods 4, 1007–1009 (2007)
Banaszynski, L. A., Chen, L.-C., Maynard-Smith, L. A., Ooi, A. G. L. & Wandless, T. J. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell 126, 995–1004 (2006)
Pradel, G. Proteins of the malaria parasite sexual stages: expression, function and potential for transmission blocking strategies. Parasitology 134, 1911–1929 (2007)
Silvestrini, F. et al. Protein export marks the early phase of gametocytogenesis of the human malaria parasite Plasmodium falciparum. Mol. Cell. Proteomics 9, 1437–1448 (2010)
Campbell, T. L., De Silva, E. K., Olszewski, K. L., Elemento, O. & Llinás, M. Identification and genome-wide prediction of DNA binding specificities for the ApiAP2 family of regulators from the malaria parasite. PLoS Pathog. 6, e1001165 (2010)
Guizetti, J. & Scherf, A. Silence, activate, poise, and switch! Mechanisms of antigenic variation in Plasmodium falciparum. Cell. Microbiol. 15, 718–726 (2013)
Pérez-Toledo, K. et al. Plasmodium falciparum heterochromatin protein 1 binds to tri-methylated histone 3 lysine 9 and is linked to mutually exclusive expression of var genes. Nucleic Acids Res. 37, 2596–2606 (2009)
Bruce, M. C., Alano, P., Duthie, S. & Carter, R. Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology 100, 191–200 (1990)
Avraham, I., Schreier, J. & Dzikowski, R. Insulator-like pairing elements regulate silencing and mutually exclusive expression in the malaria parasite Plasmodium falciparum. Proc. Natl Acad. Sci. USA 109, 52 (2012)
Sinha, A. et al. A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature http://dx.doi.org/10.1038/nature12970 (this issue)
Fivelman, Q. L. et al. Improved synchronous production of Plasmodium falciparum gametocytes in vitro. Mol. Biochem. Parasitol. 154, 119–123 (2007)
Kafsack, B. F. C., Painter, H. J. & Llinás, M. New Agilent platform DNA microarrays for transcriptome analysis of Plasmodium falciparum and Plasmodium berghei for the malaria research community. Malar. J. 11, 187 (2012)
Straimer, J. et al. Site-specific genome editing in Plasmodium falciparum using engineered zinc-finger nucleases. Nature Methods 9, 993–998 (2012)
Robinson, T. et al. Drug-resistant genotypes and multi-clonality in Plasmodium falciparum analysed by direct genome sequencing from peripheral blood of malaria patients. PLoS ONE 6, e23204 (2011)
Cortés, A., Benet, A., Cooke, B. M., Barnwell, J. W. & Reeder, J. C. Ability of Plasmodium falciparum to invade Southeast Asian ovalocytes varies between parasite lines. Blood 104, 2961–2966 (2004)
Walliker, D. et al. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science 236, 1661–1666 (1987)
Taylor, C. J. The role of two cyclic nucleotide phosphodiesterases in the sexual development of Plasmodium falciparum and Plasmodium berghei. (PhD thesis, Univ. London, 2007)
Taylor, C. J., McRobert, L. & Baker, D. A. Disruption of a Plasmodium falciparum cyclic nucleotide phosphodiesterase gene causes aberrant gametogenesis. Mol. Microbiol. 69, 110–118 (2008)
Maier, A. G., Braks, J. A. M., Waters, A. P. & Cowman, A. F. Negative selection using yeast cytosine deaminase/uracil phosphoribosyl transferase in Plasmodium falciparum for targeted gene deletion by double crossover recombination. Mol. Biochem. Parasitol. 150, 118–121 (2006)
Ménard, R. Malaria: Methods and Protocols. Methods in Molecular Biology Vol. 923, 2nd edn, 3–15 (Humana Press, Springer, 2013)
Farrell, A. et al. A DOC2 protein identified by mutational profiling is essential for apicomplexan parasite exocytosis. Science. 335, 218–221 (2012)
Calderwood, M. S., Gannoun-Zaki, L., Wellems, T. E. & Deitsch, K. W. Plasmodium falciparum var genes are regulated by two regions with separate promoters, one upstream of the coding region and a second within the intron. J. Biol. Chem. 278, 34125–34132 (2003)
Cortés, A. et al. Epigenetic silencing of Plasmodium falciparum genes linked to erythrocyte invasion. PLoS Pathog. 3, e107 (2007)
Crowley, V. M., Rovira-Graells, N., Ribas de Pouplana, L. & Cortés, A. Heterochromatin formation in bistable chromatin domains controls the epigenetic repression of clonally variant Plasmodium falciparum genes linked to erythrocyte invasion. Mol. Microbiol. 80, 391–406 (2011)
Jiang, L. et al. Epigenetic control of the variable expression of a Plasmodium falciparum receptor protein for erythrocyte invasion. Proc. Natl Acad. Sci. USA 107, 2224–2229 (2010)
SMALT - Wellcome Trust Sanger Institute. (http://www.sanger.ac.uk/resources/software/smalt/)
The Wellcome Trust Sanger Institute SRA Study ERP000190. (http://www.ebi.ac.uk/ena/data/view/ERP000190)
Acknowledgements
We would like to thank C. Klein, T. Campbell and A. Schieler for technical assistance and are grateful to O. Billker, C. Flueck, J. Kelly, C. Sutherland, A. Vaidya and A. Waters for discussion and reading of the manuscript. We would also like to thank P. Alano for providing P. falciparum clone F12, C. Taylor for providing the P. falciparum GNP-A4 clone, E. Thompson for isolating P. falciparum DNA for whole genome analysis, Z. Gorvett for assistance with confirming single nucleotide polymorphisms in gametocyte non-producing clones, M. Duraisingh for the ddFKBP tagging construct pJDD145, C. Ben Mamoun for the anti-PP2c antibody and D. Goldberg for Shld1. M.L. is funded by National Institutes of Health (NIH) grant R01 AI076276 with support from the Centre for Quantitative Biology (P50GM071508). B.F.C.K. was supported by a Howard Hughes Medical Institute fellowship of the Damon Runyon Cancer Research Foundation. D.A.B. is funded by Wellcome Trust grant ref. 094752 and European Commission FP7 ‘MALSIG’ (ref. 223044). L.G.D. is supported by a Biotechnology and Biological Sciences Research Council CASE PhD studentship with Pfizer as the Industrial partner. A.C. is funded by the Spanish Ministry of Science and Innovation grant SAF2010-20111. V.M.C. was supported by a fellowship from IRB Barcelona. T.G.C. is supported by the Medical Research Council UK (J005398). D.P.K. and S.G.C. are supported through the Wellcome Trust (098051; 090532/Z/09/Z) and the Medical Research Council UK (G0600230). C.B. is supported by the Catalan Government fellowship 2011-BP-B 00060 (AGAUR, Catalonia, Spain).
Author information
Authors and Affiliations
Contributions
M.L. managed the overall project with input from B.F.C.K., D.A.B. and A.C. B.F.C.K. generated the Δpfap2-g knockout, PfAP2-G–ddFKBP and luciferase lines and designed, performed and analysed the microarray, gel shift, luciferase and ligand-regulatable gametocytogenesis experiments. V.M.C. performed qRT–PCR validation. A.E.W. prepared Δpfap2-g sequencing libraries and together with B.F.C.K. analysed the sequencing data. D.A.B., T.G.C. and S.G.C. conceived the sequencing of gametocyte non-producer lines F12 and GNP-A4. T.G.C. analysed the gametocyte non-producer sequencing data and L.G.D. confirmed the SNPs by PCR. S.G.C. and D.P.K. carried out and supervised sequencing of gametocyte non-producer lines, respectively. A.C. and N.R.-G. generated E5 and other 3D7-B subclones and respectively supervised and performed the experiments presented in Figs. 1 and 2b, and provided the analysis presented in Supplementary Fig. 1. V.M.C. and N.R.-G. performed and A.C. supervised chromatin immunoprecipitation experiments. C.B. and A.C. generated the PfAP2-G–HA×3 line and carried out immunofluorescence assays and correlations with gametocyte formation. B.F.C.K. wrote the manuscript with major input from M.L., D.A.B. and A.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-10, Supplementary References and Supplementary Table 3. (PDF 9157 kb)
Supplementary Table 1
This table shows coding sequence mutations affecting F12, GNP-A4, and Δpfap2‐g. (XLSX 39 kb)
Supplementary Table 2
This table shows differentially expressed genes in F12 and Δpfap2-g. (XLSX 72 kb)
Rights and permissions
About this article
Cite this article
Kafsack, B., Rovira-Graells, N., Clark, T. et al. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature 507, 248–252 (2014). https://doi.org/10.1038/nature12920
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12920
This article is cited by
-
The transcription factor AP2XI-2 is a key negative regulator of Toxoplasma gondii merogony
Nature Communications (2024)
-
High-throughput analysis of the transcriptional patterns of sexual genes in malaria
Parasites & Vectors (2023)
-
Sexual differentiation in human malaria parasites is regulated by competition between phospholipid metabolism and histone methylation
Nature Microbiology (2023)
-
Extracellular vesicles could be a putative posttranscriptional regulatory mechanism that shapes intracellular RNA levels in Plasmodium falciparum
Nature Communications (2023)
-
A transcriptional network required for bradyzoite development in Toxoplasma gondii is dispensable for recrudescent disease
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.