Abstract
Preservation of neural tissue in early Cambrian arthropods has recently been demonstrated1, to a degree that segmental structures of the head can be associated with individual brain neuromeres. This association provides novel data for addressing long-standing controversies about the segmental identities of specialized head appendages in fossil taxa2,3. Here we document neuroanatomy in the head and trunk of a ‘great appendage’ arthropod, Alalcomenaeus sp., from the Chengjiang biota, southwest China, providing the most complete neuroanatomical profile known from a Cambrian animal. Micro-computed tomography reveals a configuration of one optic neuropil separate from a protocerebrum contiguous with four head ganglia, succeeded by eight contiguous ganglia in an eleven-segment trunk. Arrangements of optic neuropils, the brain and ganglia correspond most closely to the nervous system of Chelicerata of all extant arthropods, supporting the assignment of ‘great appendage’ arthropods to the chelicerate total group4,5. The position of the deutocerebral neuromere aligns with the insertion of the great appendage, indicating its deutocerebral innervation and corroborating a homology between the ‘great appendage’ and chelicera indicated by morphological similarities4,6,7. Alalcomenaeus and Fuxianhuia protensa1 demonstrate that the two main configurations of the brain observed in modern arthropods, those of Chelicerata and Mandibulata, respectively8, had evolved by the early Cambrian.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ma, X., Hou, X., Edgecombe, G. D. & Strausfeld, N. J. Complex brain and optic lobes in an early Cambrian arthropod. Nature 490, 258–261 (2012)
Budd, G. E. A palaeontological solution to the arthropod head problem. Nature 417, 271–275 (2002)
Yang, J., Ortega-Hernández, J., Butterfield, N. J. & Zhang, X. Specialized appendages in fuxianhuiids and the head organization of early arthropods. Nature 494, 468–471 (2013)
Cotton, T. J. & Braddy, S. J. The phylogeny of arachnomorph arthropods and the origin of the Chelicerata. Trans. R. Soc. Edinb. Earth Sci. 94, 169–193 (2003)
Stein, M., Budd, G. E., Peel, J. S. & Harper, D. A. T. Arthroaspis n. gen., a common element of the Sirius Passet Lagerstätte (Cambrian, North Greenland), sheds light on trilobite ancestry. BMC Evol. Biol. 13, 99 (2013)
Chen, J., Waloszek, D. & Maas, A. A new ‘great-appendage’ arthropod from the Lower Cambrian of China and homology of chelicerate chelicerae and raptorial antero-ventral appendages. Lethaia 37, 3–20 (2004)
Haug, J. T., Waloszek, D., Maas, A., Liu, Y. & Haug, C. Functional morphology, ontogeny and evolution of mantis shrimp-like predators in the Cambrian. Palaeontology 55, 369–399 (2012)
Strausfeld, N. J. Arthropod Brains: Evolution, Functional Elegance, and Historical Significance (Harvard Univ. Press, 2012)
Hou, X. & Bergström, J. Arthropods of the Lower Cambrian Chengjiang fauna, southwest China. Fossils and Strata 45, 1–116 (1997)
Legg, D. A., Sutton, M. D., Edgecombe, G. D. & Caron, J.-B. Cambrian bivalved arthropod reveals origins of arthrodisation. Proc. R. Soc. Lond. B 279, 4699–4704 (2012)
Edgecombe, G. D., García-Bellido, D. C. & Paterson, J. R. A new leanchoiliid megacheiran arthropod from the lower Cambrian Emu Bay Shale, South Australia. Acta Palaeontol. Pol. 56, 385–400 (2011)
Haug, J. T., Briggs, D. E. G. & Haug, C. Morphology and function in the Cambrian Burgess Shale megacheiran arthropod Leanchoilia superlata and the application of a descriptive matrix. BMC Evol. Biol. 12, 162 (2012)
Hou, X.-G. et al. The Cambrian Fossils of Chengjiang, China: The Flowering of Early Animal Life (Blackwell, 2004)
Liu, Y., Hou, X. & Bergström, J. Chengjiang arthropod Leanchoilia illecebrosa (Hou, 1987) reconsidered. GFF 129, 263–272 (2007)
Briggs, D. E. G. & Collins, D. The arthropod Alalcomenaeus cambricus Simonetta, from the Middle Cambrian Burgess Shale of British Columbia. Palaeontology 42, 953–977 (1999)
Brenneis, G. & Richter, S. Architecture of the nervous system in Mystacocarida (Arthropoda, Crustacea)—an immunohistochemical study and 3D reconstruction. J. Morphol. 271, 169–189 (2010)
Damen, W. G., Hausdorf, M., Seyfarth, E. A. & Tautz, D. A conserved mode of head segmentation in arthropods revealed by the expression pattern of Hox genes in a spider. Proc. Natl Acad. Sci. USA 95, 10665–10670 (1998)
García-Bellido, D. C. & Collins, D. Reassessment of the genus Leanchoilia (Arthropoda, Arachnomorpha) from the Middle Cambrian Burgess Shale, British Columbia, Canada. Palaeontology 50, 693–709 (2007)
Richter, S., Stein, M., Frase, T. & Szucsich, N. U. in Arthropod Biology and Evolution (eds Minelli A., Boxshall G. & Fusco G. ) The Arthropod Head 223–240 (Springer, 2013)
Butterfield, N. J. Leanchoilia guts and the interpretation of three-dimensional structures in Burgess Shale-type fossils. Paleobiology 28, 155–171 (2002)
Lehmann, T., Hess, M. & Melzer, R. R. Wiring a periscope - ocelli, retinula axons, visual neuropils and the ancestrality of sea spiders. PLoS ONE 7, e30474 (2012)
Harzsch, S. et al. Evolution of arthropod visual systems: development of the eyes and central visual pathways in the horseshoe crab Limulus polyphemus Linnaeus, 1758 (Chelicerata, Xiphosura). Dev. Dyn. 235, 2641–2655 (2006)
Strausfeld, N. J., Weltzien, P. & Barth, F. G. Two visual systems in one brain: neuropil serving the principal eyes of the spider Cupiennius salei. J. Comp. Neurol. 328, 63–75 (1993)
Strausfeld, N. J. & Andrew, D. R. A new view of insect-crustacean relationships. I. Inferences from neural cladistics and comparative neuroanatomy. Arthropod Struct. Dev. 40, 276–288 (2011)
Zeil, J. Sexual dimorphism in the visual system of flies: the divided brain of male Bibionidae (Diptera). Cell Tissue Res. 229, 591–610 (1983)
Lin, C. & Strausfeld, N. J. A precocious adult visual center in the larva defines the unique optic lobe of the split-eyed whirligig beetle Dineutus sublineatus. Front. Zool. 10, 7 (2013)
Schoenemann, B. & Clarkson, E. N. K. The eyes of Leanchoilia. Lethaia 45, 524–531 (2012)
Eriksson, M. E. & Terfelt, F. Exceptionally preserved Cambrian trilobite digestive system revealed in 3D by synchrotron-radiation X-ray tomographic microscopy. PLoS ONE 7, e35625 (2012)
Eriksson, M. E., Terfelt, F., Elofsson, R. & Marone, F. Internal soft-tissue anatomy of Cambrian ‘Orsten’ arthropods as revealed by synchrotron X-ray tomographic microscopy. PLoS ONE 7, e42582 (2012)
Acknowledgements
We thank N. Shimobayashi, H. Maeda, and T. Kogiso for arranging and performing EDXRF analyses, and D. Andrew for advice on cladistics. This work was supported by grants from the Natural Science Foundation of China (no. 40730211), Research in Education and Science from the Government of Japan (no. 21740370), a Leverhulme Trust Research Project Grant (F/00 696/T), by the Center for Insect Science, University of Arizona, and a grant from the Air Force Research Laboratories (FA8651-10-1-0001) to N.J.S.
Author information
Authors and Affiliations
Contributions
The project was conceived by G.T. Fossil data were analysed by all authors. G.D.E., N.J.S. and X.M. composed the text.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
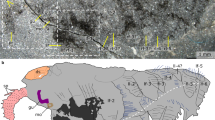
Extended Data Figure 1 Cephalic region of Alalcomenaeus sp. YKLP 11075
All in dorsal view, composites of part and counterpart (upper left). Second left to right: CT scan (green); EDXRF Fe (red); superimposition of CT and EDXRF Fe. Lower row, left to right: EDXRF Cu (blue); superimposition of CT and EDXRF Cu; superimposition of EDXRF Fe and EDXRF Cu; superimposition of all scans. C1, first post-GA neuropil = tritocerebrum (tri); C2, second post-GA neuropil; GA, great appendage neuropil = deutocerebrum (deu); on1, first optic neuropil; pr, protocerebrum.
Extended Data Figure 2 Arthropod relationships based on neuroanatomical characters.
Strict consensus of 34 shortest cladograms based on 145 characters in Supplementary Information Table 2.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-2, Phylogenetic Methods and Supplementary References. (PDF 249 kb)
Supplementary Data
This zipped file contains characters coded in phylogenetic analysis (in nexus format, it can be opened in freeware such as Mesquite and Nexus Data Editor). (ZIP 3 kb)
Rights and permissions
About this article
Cite this article
Tanaka, G., Hou, X., Ma, X. et al. Chelicerate neural ground pattern in a Cambrian great appendage arthropod. Nature 502, 364–367 (2013). https://doi.org/10.1038/nature12520
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12520
This article is cited by
-
The median eyes of trilobites
Scientific Reports (2023)
-
A Middle Ordovician Burgess Shale-type fauna from Castle Bank, Wales (UK)
Nature Ecology & Evolution (2023)
-
The visual pathway in sea spiders (Pycnogonida) displays a simple serial layout with similarities to the median eye pathway in horseshoe crabs
BMC Biology (2022)
-
Unparalleled details of soft tissues in a Cretaceous ant
BMC Ecology and Evolution (2022)
-
Ordovician opabiniid-like animals and the role of the proboscis in euarthropod head evolution
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.