Abstract
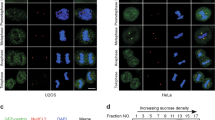
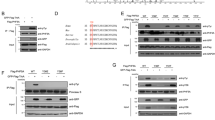
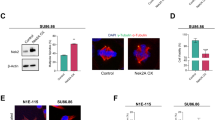
Centrosome duplication is critical for cell division, and genome instability can result if duplication is not restricted to a single round per cell cycle. Centrosome duplication is controlled in part by CP110, a centriolar protein that positively regulates centriole duplication while restricting centriole elongation and ciliogenesis. Maintenance of normal CP110 levels is essential, as excessive CP110 drives centrosome over-duplication and suppresses ciliogenesis, whereas its depletion inhibits centriole amplification and leads to highly elongated centrioles and aberrant assembly of cilia in growing cells1,2. CP110 levels are tightly controlled, partly through ubiquitination by the ubiquitin ligase complex SCFcyclin F during G2 and M phases of the cell cycle3. Here, using human cells, we report a new mechanism for the regulation of centrosome duplication that requires USP33, a deubiquitinating enzyme that is able to regulate CP110 levels. USP33 interacts with CP110 and localizes to centrioles primarily in S and G2/M phases, the periods during which centrioles duplicate and elongate. USP33 potently and specifically deubiquitinates CP110, but not other cyclin-F substrates. USP33 activity antagonizes SCFcyclin F-mediated ubiquitination and promotes the generation of supernumerary centriolar foci, whereas ablation of USP33 destabilizes CP110 and thereby inhibits centrosome amplification and mitotic defects. To our knowledge, we have identified the first centriolar deubiquitinating enzyme whose expression regulates centrosome homeostasis by countering cyclin-F-mediated destruction of a key substrate. Our results point towards potential therapeutic strategies for inhibiting tumorigenesis associated with centrosome amplification.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chen, Z., Indjeian, V. B., McManus, M., Wang, L. & Dynlacht, B. D. CP110, a cell cycle-dependent CDK substrate, regulates centrosome duplication in human cells. Dev. Cell 3, 339–350 (2002)
Spektor, A., Tsang, W. Y., Khoo, D. & Dynlacht, B. D. Cep97 and CP110 suppress a cilia assembly program. Cell 130, 678–690 (2007)
D’Angiolella, V. et al. SCFCyclin F controls centrosome homeostasis and mitotic fidelity through CP110 degradation. Nature 466, 138–142 (2010)
Li, J. et al. Neurl4, a novel daughter centriole protein, prevents formation of ectopic microtubule organizing centres. EMBO Rep. 13, 547–553 (2012)
Sowa, M. E., Bennett, E. J., Gygi, S. P. & Harper, J. W. Defining the human deubiquitinating enzyme interaction landscape. Cell 138, 389–403 (2009)
Li, Z. et al. Ubiquitination of a novel deubiquitinating enzyme requires direct binding to von Hippel-Lindau tumor suppressor protein. J. Biol. Chem. 277, 4656–4662 (2002)
Li, Z. et al. Identification of a deubiquitinating enzyme subfamily as substrates of the von Hippel–Lindau tumor suppressor. Biochem. Biophys. Res. Commun. 294, 700–709 (2002)
Curcio-Morelli, C. et al. Deubiquitination of type 2 iodothyronine deiodinase by von Hippel-Lindau protein-interacting deubiquitinating enzymes regulates thyroid hormone activation. J. Clin. Invest. 112, 189–196 (2003)
Thorne, C., Eccles, R. L., Coulson, J. M., Urbe, S. & Clague, M. J. Isoform-specific localization of the deubiquitinase USP33 to the Golgi apparatus. Traffic 12, 1563–1574 (2011)
D’Angiolella, V. et al. Cyclin F-mediated degradation of ribonucleotide reductase M2 controls genome integrity and DNA repair. Cell 149, 1023–1034 (2012)
Berthouze, M., Venkataramanan, V., Li, Y. & Shenoy, S. K. The deubiquitinases USP33 and USP20 coordinate β2 adrenergic receptor recycling and resensitization. EMBO J. 28, 1684–1696 (2009)
Chan, J. Y. A clinical overview of centrosome amplification in human cancers. Int. J. Biol. Sci. 7, 1122–1144 (2011)
Ganem, N. J., Godinho, S. A. & Pellman, D. A mechanism linking extra centrosomes to chromosomal instability. Nature 460, 278–282 (2009)
Buchholz, M. et al. Transcriptome analysis of microdissected pancreatic intraepithelial neoplastic lesions. Oncogene 24, 6626–6636 (2005)
Badea, L., Herlea, V., Dima, S. O., Dumitrascu, T. & Popescu, I. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepatogastroenterology 55, 2016–2027 (2008)
Sato, N. et al. Centrosome abnormalities in pancreatic ductal carcinoma. Clin. Cancer Res. 5, 963–970 (1999)
Sato, N. et al. Correlation between centrosome abnormalities and chromosomal instability in human pancreatic cancer cells. Cancer Genet. Cytogenet. 126, 13–19 (2001)
Shono, M. et al. Stepwise progression of centrosome defects associated with local tumor growth and metastatic process of human pancreatic carcinoma cells transplanted orthotopically into nude mice. Lab. Invest. 81, 945–952 (2001)
Cohen, P. & Tcherpakov, M. Will the ubiquitin system furnish as many drug targets as protein kinases? Cell 143, 686–693 (2010)
Sippl, W., Collura, V. & Colland, F. Ubiquitin-specific proteases as cancer drug targets. Future Oncol. 7, 619–632 (2011)
Tansey, W. P. Detection of ubiquitylated proteins in mammalian cells. Cold Spring Harb. Protoc.. http://dx.doi.org/10.1101/pdb.prot4616 (2006)
Sabbaghian, M. S. et al. Levels of elevated circulating endothelial cell decline after tumor resection in patients with pancreatic ductal adenocarcinoma. Anticancer Res. 30, 2911–2917 (2010)
Seeley, E. S., Carriere, C., Goetze, T., Longnecker, D. S. & Korc, M. Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Res. 69, 422–430 (2009)
Acknowledgements
We thank C. Hajdu, G. Miller, L. Chiriboga, H. Court, C. Zambirinis, E. Bekes, L. Taylor, S. Krauter, M. Philips and D. Bar-Sagi for help with staining and analysis of pancreatic cancer samples. We thank F. Liang, Y. Deng, C. Petzold and K. Dancel for help with correlative electron microscopy. We thank J. Jastrab and S. Vijayakumar for initial experiments pertaining to the USP20–CP110 interaction. We thank J. Pollack for providing pancreatic cell lines. We thank G. Wu, S. Shenoy, R. Baer and T. Huang for providing plasmids. We thank members of the Dynlacht laboratory and T. Huang for advice. B.D.D. was supported by National Institutes of Health grant 5R01HD069647-02 and March of Dimes grant FY11-432. M.P. is an Investigator with the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
J.L., V.D’A., S.K., T.K., E.I.C., M.P. and B.D.D. performed all biochemical experiments and analyses of deubiquitination and centrosome phenotypes. E.S.S., W.F. and J.L. performed pancreatic tissue studies. J. L. and B.D.D. wrote the manuscript. B.D.D. coordinated the study and oversaw all experiments. All authors discussed the results and commented on and assisted in revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-9. (PDF 2557 kb)
Rights and permissions
About this article
Cite this article
Li, J., D’Angiolella, V., Seeley, E. et al. USP33 regulates centrosome biogenesis via deubiquitination of the centriolar protein CP110. Nature 495, 255–259 (2013). https://doi.org/10.1038/nature11941
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11941
This article is cited by
-
CCP5 and CCP6 retain CP110 and negatively regulate ciliogenesis
BMC Biology (2023)
-
DNAJA2 deficiency activates cGAS-STING pathway via the induction of aberrant mitosis and chromosome instability
Nature Communications (2023)
-
Appearing and disappearing acts of cilia
Journal of Biosciences (2023)
-
Aggresome assembly at the centrosome is driven by CP110–CEP97–CEP290 and centriolar satellites
Nature Cell Biology (2022)
-
Deubiquitinating enzyme USP33 restrains docetaxel-induced apoptosis via stabilising the phosphatase DUSP1 in prostate cancer
Cell Death & Differentiation (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.