Abstract
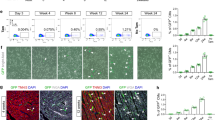
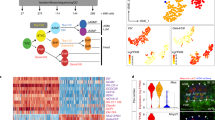
As vertebrate embryos develop to adulthood, their organs undergo marked changes in size and tissue architecture. The heart acquires muscle mass and matures structurally to fulfil increasing circulatory needs, a process that is incompletely understood. Here we used multicolour clonal analysis to define the contributions of individual cardiomyocytes as the zebrafish heart undergoes morphogenesis from a primitive embryonic structure into its complex adult form. We find that the single-cardiomyocyte-thick wall of the juvenile ventricle forms by lateral expansion of several dozen cardiomyocytes into muscle patches of variable sizes and shapes. As juvenile zebrafish mature into adults, this structure becomes fully enveloped by a new lineage of cortical muscle. Adult cortical muscle originates from a small number of cardiomyocytes—an average of approximately eight per animal—that display clonal dominance reminiscent of stem cell populations. Cortical cardiomyocytes initially emerge from internal myofibres that in rare events breach the juvenile ventricular wall, and then expand over the surface. Our results illuminate the dynamic proliferative behaviours that generate adult cardiac structure, revealing clonal dominance as a key mechanism that shapes a vertebrate organ.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Livet, J. et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450, 56–62 (2007)
Zhou, Y. et al. Latent TGF-β binding protein 3 identifies a second heart field in zebrafish. Nature 474, 645–648 (2011)
Kikuchi, K. et al. tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development 138, 2895–2902 (2011)
Cai, C. L. et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell 5, 877–889 (2003)
Zhou, B. et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454, 109–113 (2008)
Meilhac, S. M., Esner, M., Kelly, R. G., Nicolas, J. F. & Buckingham, M. E. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev. Cell 6, 685–698 (2004)
Keegan, B. R., Meyer, D. & Yelon, D. Organization of cardiac chamber progenitors in the zebrafish blastula. Development 131, 3081–3091 (2004)
Stainier, D. Y., Lee, R. K. & Fishman, M. C. Cardiovascular development in the zebrafish. I. Myocardial fate map and heart tube formation. Development 119, 31–40 (1993)
Mikawa, T., Borisov, A., Brown, A. M. & Fischman, D. A. Clonal analysis of cardiac morphogenesis in the chicken embryo using a replication-defective retrovirus. I. Formation of the ventricular myocardium. Dev. Dyn. 193, 11–23 (1992)
Meilhac, S. M. et al. A retrospective clonal analysis of the myocardium reveals two phases of clonal growth in the developing mouse heart. Development 130, 3877–3889 (2003)
Kikuchi, K. et al. Primary contribution to zebrafish heart regeneration by gata4+ cardiomyocytes. Nature 464, 601–605 (2010)
de Pater, E. et al. Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart. Development 136, 1633–1641 (2009)
Auman, H. J. et al. Functional modulation of cardiac form through regionally confined cell shape changes. PLoS Biol. 5, e53 (2007)
Hami, D., Grimes, A. C., Tsai, H. J. & Kirby, M. L. Zebrafish cardiac development requires a conserved secondary heart field. Development 138, 2389–2398 (2011)
Lazic, S. & Scott, I. C. Mef2cb regulates late myocardial cell addition from a second heart field-like population of progenitors in zebrafish. Dev. Biol. 354, 123–133 (2011)
Hu, N., Yost, H. J. & Clark, E. B. Cardiac morphology and blood pressure in the adult zebrafish. Anat. Rec. 264, 1–12 (2001)
Liu, J. et al. A dual role for ErbB2 signaling in cardiac trabeculation. Development 137, 3867–3875 (2010)
Poss, K. D., Wilson, L. G. & Keating, M. T. Heart regeneration in zebrafish. Science 298, 2188–2190 (2002)
Wang, J. et al. The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development 138, 3421–3430 (2011)
Jopling, C. et al. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 464, 606–609 (2010)
Snippert, H. J. et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144 (2010)
Klein, A. M., Nakagawa, T., Ichikawa, R., Yoshida, S. & Simons, B. D. Mouse germ line stem cells undergo rapid and stochastic turnover. Cell Stem Cell 7, 214–224 (2010)
Lopez-Garcia, C., Klein, A. M., Simons, B. D. & Winton, D. J. Intestinal stem cell replacement follows a pattern of neutral drift. Science 330, 822–825 (2010)
Doupé, D. P., Klein, A. M., Simons, B. D. & Jones, P. H. The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev. Cell 18, 317–323 (2010)
Burns, C. G. et al. High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nature Chem. Biol. 1, 263–264 (2005)
Cooper, M. S. et al. Visualizing morphogenesis in transgenic zebrafish embryos using BODIPY TR methyl ester dye as a vital counterstain for GFP. Dev. Dyn. 232, 359–368 (2005)
Kikuchi, K. et al. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev. Cell 20, 397–404 (2011)
Acknowledgements
We thank K. Kikuchi for generating cmlc2:CreER animals and for advice; J. Burris, A. Eastes, P. Williams and N. Blake for zebrafish care; A. Dickson for artwork; B. Hogan and Poss laboratory members for comments on the manuscript; and S. Johnson and Y. Gao for imaging advice. V.G. was supported by a National Heart, Lung, and Blood Institute (NHLBI) Medical Scientist Training Program supplement. K.D.P. is an Early Career Scientist of the Howard Hughes Medical Institute. This work was supported by grants from NHLBI (HL081674) and American Heart Association to K.D.P.
Author information
Authors and Affiliations
Contributions
V.G. and K.D.P. designed experimental strategy, analysed data, and prepared the manuscript. V.G. performed all of the experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-10 (PDF 21688 kb)
Rights and permissions
About this article
Cite this article
Gupta, V., Poss, K. Clonally dominant cardiomyocytes direct heart morphogenesis. Nature 484, 479–484 (2012). https://doi.org/10.1038/nature11045
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11045
This article is cited by
-
Regeneration of the heart: from molecular mechanisms to clinical therapeutics
Military Medical Research (2023)
-
hapln1a+ cells guide coronary growth during heart morphogenesis and regeneration
Nature Communications (2023)
-
Multicolor strategies for investigating clonal expansion and tissue plasticity
Cellular and Molecular Life Sciences (2022)
-
Postnatal state transition of cardiomyocyte as a primary step in heart maturation
Protein & Cell (2022)
-
Molecular regulation of myocardial proliferation and regeneration
Cell Regeneration (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.