Abstract
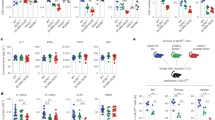
NLRs (nucleotide-binding domain leucine-rich-repeat-containing receptors; NOD-like receptors) are a class of pattern recognition receptor (PRR) that respond to host perturbation from either infectious agents or cellular stress1,2. The function of most NLR family members has not been characterized and their role in instructing adaptive immune responses remains unclear2,3. NLRP10 (also known as PYNOD, NALP10, PAN5 and NOD8) is the only NLR lacking the putative ligand-binding leucine-rich-repeat domain, and has been postulated to be a negative regulator of other NLR members, including NLRP3 (refs 4–6). We did not find evidence that NLRP10 functions through an inflammasome to regulate caspase-1 activity nor that it regulates other inflammasomes. Instead, Nlrp10−/− mice had a profound defect in helper T-cell-driven immune responses to a diverse array of adjuvants, including lipopolysaccharide, aluminium hydroxide and complete Freund’s adjuvant. Adaptive immunity was impaired in the absence of NLRP10 because of a dendritic cell (DC) intrinsic defect in emigration from inflamed tissues, whereas upregulation of DC costimulatory molecules and chemotaxis to CCR7-dependent and -independent ligands remained intact. The loss of antigen transport to the draining lymph nodes by a subset of migratory DCs resulted in an almost absolute loss in naive CD4+ T-cell priming, highlighting the critical link between diverse innate immune stimulation, NLRP10 activity and the immune function of mature DCs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
25 November 2015
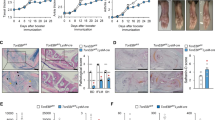
Nature 484, 510–513 (2012); 10.1038/nature11012 In this Letter, we reported that NLRP10-deficient mice had no defect in inflammasome function in macrophages or dendritic cells (DCs). Instead, a loss of T-cell-dependent immune responses was seen in these mice secondary to a defect in DC migration. Wehave since noticed a change in the phenotype of the NLRP10-knockout mice involving DC migration, after backcrossing them onto backgrounds such as FVB or BALB/c (see Supplementary Methods).
References
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010)
Williams, A., Flavell, R. A. & Eisenbarth, S. C. The role of NOD-like receptors in shaping adaptive immunity. Curr. Opin. Immunol. 22, 34–40 (2010)
Medzhitov, R. & Janeway, C. A., Jr Innate immune induction of the adaptive immune response. Cold Spring Harb. Symp. Quant. Biol. 64, 429–436 (1999)
Imamura, R. et al. Anti-inflammatory activity of PYNOD and its mechanism in humans and mice. J. Immunol. 184, 5874–5884 (2010)
Inohara, N. & Nunez, G. NODs: intracellular proteins involved in inflammation and apoptosis. Nature Rev. Immunol. 3, 371–382 (2003)
Wang, Y. et al. PYNOD, a novel Apaf-1/CED4-like protein is an inhibitor of ASC and caspase-1. Int. Immunol. 16, 777–786 (2004)
Eisenbarth, S. C., Colegio, O. R., O’Connor, W., Sutterwala, F. S. & Flavell, R. A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453, 1122–1126 (2008)
Li, H., Willingham, S. B., Ting, J. P. & Re, F. Cutting edge: inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J. Immunol. 181, 17–21 (2008)
Gris, D. et al. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J. Immunol. 185, 974–981 (2010)
Eisenbarth, S. C. et al. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 196, 1645–1651 (2002)
Bachmann, M. F., Hengartner, H. & Zinkernagel, R. M. T helper cell-independent neutralizing B cell response against vesicular stomatitis virus: role of antigen patterns in B cell induction? Eur. J. Immunol. 25, 3445–3451 (1995)
Palm, N. W. & Medzhitov, R. Immunostimulatory activity of haptenated proteins. Proc. Natl Acad. Sci. USA 106, 4782–4787 (2009)
Banchereau, J. & Steinman, R. M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998)
Ohl, L. et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 21, 279–288 (2004)
Martin-Fontecha, A. et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 198, 615–621 (2003)
Saeki, H., Moore, A. M., Brown, M. J. & Hwang, S. T. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J. Immunol. 162, 2472–2475 (1999)
Itano, A. A. et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity 19, 47–57 (2003)
Jakubzick, C. et al. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J. Exp. Med. 205, 2839–2850 (2008)
Edelson, B. T. et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8α+ conventional dendritic cells. J. Exp. Med. 207, 823–836 (2010)
Ginhoux, F. et al. The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 206, 3115–3130 (2009)
Plantinga, M., Hammad, H. & Lambrecht, B. N. Origin and functional specializations of DC subsets in the lung. Eur. J. Immunol. 40, 2112–2118 (2010)
Cyster, J. G. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 23, 127–159 (2005)
Czeloth, N., Bernhardt, G., Hofmann, F., Genth, H. & Forster, R. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J. Immunol. 175, 2960–2967 (2005)
Dieu, M. C. et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J. Exp. Med. 188, 373–386 (1998)
Gunn, M. D. et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 189, 451–460 (1999)
Sallusto, F. et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 28, 2760–2769 (1998)
Noben-Trauth, N. et al. An interleukin 4 (IL-4)-independent pathway for CD4+ T cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc. Natl Acad. Sci. USA 94, 10838–10843 (1997)
Zheng, B., Berrie, C. P., Corda, D. & Farquhar, M. G. GDE1/MIR16 is a glycerophosphoinositol phosphodiesterase regulated by stimulation of G protein-coupled receptors. Proc. Natl Acad. Sci. USA 100, 1745–1750 (2003)
Arthur, J. C. et al. Cutting edge: NLRP12 controls dendritic and myeloid cell migration to affect contact hypersensitivity. J. Immunol. 185, 4515–4519 (2010)
Ippagunta, S. K. et al. The inflammasome adaptor ASC regulates the function of adaptive immune cells by controlling Dock2-mediated Rac activation and actin polymerization. Nature Immunol. 12, 1010–1016 (2011)
Sutterwala, F. S. et al. Critical role for NALP3/CIAS1/cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24, 317–327 (2006)
Cohn, L., Homer, R. J., Niu, N. & Bottomly, K. T helper 1 cells and interferon γ regulate allergic airway inflammation and mucus production. J. Exp. Med. 190, 1309–1318 (1999)
Laouar, Y. et al. TGF-β signaling in dendritic cells is a prerequisite for the control of autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 105, 10865–10870 (2008)
Lutz, M. B. et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 (1999)
Jakubzick, C., Helft, J., Kaplan, T. J. & Randolph, G. J. Optimization of methods to study pulmonary dendritic cell migration reveals distinct capacities of DC subsets to acquire soluble versus particulate antigen. J. Immunol. Methods 337, 121–131 (2008)
Edgar, R., Domrachev, M. & Lash, A. E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002)
Acknowledgements
We would like to thank R. Medzhitov and M. Albert for discussion and review of this manuscript, and F. Duffy for assistance with manuscript preparation. S.C.E. was supported by T32HL007974, K08AI085038 and Yale CTSA (UL1 RR024139). O.R.C. was supported by the Damon Runyon Cancer Research Foundation (DRG 108-09), the Yale CTSA (UL1 RR024139 and 5KL2RR024138), the Yale SPORE in Skin Cancer (1 P50 CA121974) and the Dermatology Foundation. E.E. is supported by Cancer Research Institute, the American Physicians for Medicine in Israel Foundation, and the United States-Israel binational Foundation grant. A.M.H., D.G.G. and in vivo imaging were supported by Yale Rheumatologic Disease Research Core Center P30AR053495. F.S.S. was supported by R01AI087630 and an Edward Mallinckrodt, Jr. Foundation scholarship. A.W. was a Howard Hughes fellow and R.A.F. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
S.C.E. and A.W. wrote the manuscript, designed, performed and interpreted experiments with technical assistance from L.X., F.S.S. generated Nlrp10−/− mice, S.J. performed in vitro inflammasome activation, O.R.C. and J.H.-M. assisted with trans-well assays and performed real-time PCR, L.A.Z. assisted with EAE experiments, T.S. assisted with TNP immunizations, A.R. assisted with intravenous LPS experiments, E.E. provided technical assistance with DC isolations, C.A.T. performed immunofluorescence experiments, H.M. and S.H.K. performed array analysis, D.G. and A.M.H. performed intravital microscopy and quantification. R.A.F. assisted in experimental design and interpretation. S.C.E. and R.A.F. directed the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-13. (PDF 1482 kb)
Supplementary Movie 1
This movie shows intravital imaging of WT and Nlrp10-/- dendritic cells in the skin. After an overnight stimulation with 1 μg/ml of LPS, 0.5 x 105 WT DCs labelled with CMTMR (Red) and 0.5 x 105 Nlrp10-/- DCs cells labelled with CFSE (Green) were mixed with an equal number of unlabelled WT and Nlrp10-/- DCs and co-injected intradermally with LPS into the ears of WT mice. Intravital two-photon laser scanning microscopy was performed four hours later. A blue emission resulting from second harmonic generation of collagen fibres highlights the epidermal junction. Images of skin adjacent to the injection site were collected every 30 seconds over the course of 60 minutes. The rendered movie represents a maximum intensity projection of a stack of 15 optical sections of a 400 um field of view. Movie is representative of three independent experiments. (MOV 4592 kb)
Supplementary Movie 2
This movie shows intravital microscopy of WT and Nlrp10-/- dendritic cells in the skin. After an overnight stimulation with 1 μg/ml of LPS, 1 x 105 WT DCs labelled with CMTMR (Red) and 1 x 105 Nlrp10-/- DCs cells labelled with CFSE (Green) were co-injected intradermally with LPS into the ears of WT mice and imaged as in Movie 1 four hours later. Images were collected every 30 seconds over the course of 80 minutes and the rendered movie represents a maximum intensity projection of a stack of 15 optical sections digitally zoomed to a 350 um field of view. Importantly, swapping the dye label between the WT and Nlrp10-/- DCs did not affect the results obtained by 2-photon microscopy (data not shown). (MOV 6740 kb)
Rights and permissions
About this article
Cite this article
Eisenbarth, S., Williams, A., Colegio, O. et al. NLRP10 is a NOD-like receptor essential to initiate adaptive immunity by dendritic cells. Nature 484, 510–513 (2012). https://doi.org/10.1038/nature11012
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11012
This article is cited by
-
NLRP10 promotes AGEs-induced NLRP1 and NLRP3 inflammasome activation via ROS/MAPK/NF-κB signaling in human periodontal ligament cells
Odontology (2024)
-
An inventory of adjuvants used for vaccination in horses: the past, the present and the future
Veterinary Research (2023)
-
The NLR gene family: from discovery to present day
Nature Reviews Immunology (2023)
-
Epithelial Nlrp10 inflammasome mediates protection against intestinal autoinflammation
Nature Immunology (2023)
-
Role of Microgliosis and NLRP3 Inflammasome in Parkinson’s Disease Pathogenesis and Therapy
Cellular and Molecular Neurobiology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.