Abstract
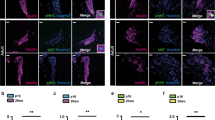
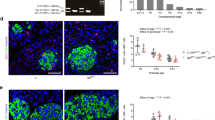
Determining the signalling pathways that direct tissue expansion is a principal goal of regenerative biology. Vigorous pancreatic β-cell replication in juvenile mice and humans declines with age, and elucidating the basis for this decay may reveal strategies for inducing β-cell expansion, a long-sought goal for diabetes therapy. Here we show that platelet-derived growth factor receptor (Pdgfr) signalling controls age-dependent β-cell proliferation in mouse and human pancreatic islets. With age, declining β-cell Pdgfr levels were accompanied by reductions in β-cell enhancer of zeste homologue 2 (Ezh2) levels and β-cell replication. Conditional inactivation of the Pdgfra gene in β-cells accelerated these changes, preventing mouse neonatal β-cell expansion and adult β-cell regeneration. Targeted human PDGFR-α activation in mouse β-cells stimulated Erk1/2 phosphorylation, leading to Ezh2-dependent expansion of adult β-cells. Adult human islets lack PDGF signalling competence, but exposure of juvenile human islets to PDGF-AA stimulated β-cell proliferation. The discovery of a conserved pathway controlling age-dependent β-cell proliferation indicates new strategies for β-cell expansion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Heit, J. J., Karnik, S. K. & Kim, S. K. Intrinsic regulators of pancreatic β-cell proliferation. Annu. Rev. Cell Dev. Biol. 22, 311–338 (2006)
Vasavada, R. C. et al. Growth factors and β-cell replication. Int. J. Biochem. Cell Biol. 38, 931–950 (2006)
Vasavada, R. C. et al. Targeted expression of placental lactogen in the β-cells of transgenic mice results in β-cell proliferation, islet mass augmentation, and hypoglycemia. J. Biol. Chem. 275, 15399–15406 (2000)
Garcia-Ocana, A. et al. Hepatocyte growth factor overexpression in the islet of transgenic mice increases β-cell proliferation, enhances islet mass, and induces mild hypoglycemia. J. Biol. Chem. 275, 1226–1232 (2000)
Beattie, G. M. et al. A novel approach to increase human islet cell mass while preserving β-cell function. Diabetes 51, 3435–3439 (2002)
Parnaud, G. et al. Proliferation of sorted human and rat β-cells. Diabetologia 51, 91–100 (2008)
Dor, Y. et al. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41–46 (2004)
Kushner, J. A. et al. Cyclins D2 and D1 are essential for postnatal pancreatic β-cell growth. Mol. Cell. Biol. 25, 3752–3762 (2005)
Teta, M. et al. Very slow turnover of β-cells in aged adult mice. Diabetes 54, 2557–2567 (2005)
Meier, J. J. et al. β-Cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes 57, 1584–1594 (2008)
Krishnamurthy, J. et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature 443, 453–457 (2006)
Zindy, F. et al. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene 15, 203–211 (1997)
Chen, H. et al. Polycomb protein Ezh2 regulates pancreatic β-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev. 23, 975–985 (2009)
Cao, R. et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298, 1039–1043 (2002)
van der Vlag, J. & Otte, A. P. Transcriptional repression mediated by the human polycomb-group protein EED involves histone deacetylation. Nature Genet. 23, 474–478 (1999)
Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 103, 211–225 (2000)
Swenne, I. et al. Effects of platelet-derived growth factor and somatomedin-C/insulin-like growth factor I on the deoxyribonucleic acid replication of fetal rat islets of Langerhans in tissue culture. Endocrinology 122, 214–218 (1988)
Welsh, M. et al. Coexpression of the platelet-derived growth factor (PDGF) B chain and the PDGF beta receptor in isolated pancreatic islet cells stimulates DNA synthesis. Proc. Natl Acad. Sci. USA 87, 5807–5811 (1990)
Su, I. et al. Polycomb group protein Ezh2 controls actin polymerization and cell signaling. Cell 121, 425–436 (2005)
Mendel, D. B. et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin. Cancer Res. 9, 327–337 (2003)
Hilberg, F. et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 68, 4774–4782 (2008)
Moenning, A. et al. Sustained platelet-derived growth factor receptor α signaling in osteoblasts results in craniosynostosis by overactivating the phospholipase C-γ pathway. Mol. Cell. Biol. 29, 881–891 (2009)
Duncia, J. V. et al. MEK inhibitors: the chemistry and biological activity of U0126, its analogs, and cyclization products. Bioorg. Med. Chem. Lett. 8, 2839–2844 (1998)
Uhrbom, L., Nerio, E. & Holland, E. C. Dissecting tumor maintenance requirements using bioluminescence imaging of cell proliferation in a mouse glioma model. Nature Med. 10, 1257–1260 (2004)
Furstoss, O., Manes, G. & Roche, S. Cyclin E and cyclin A are likely targets of Src for PDGF-induced DNA synthesis in fibroblasts. FEBS Lett. 526, 82–86 (2002)
Bracken, A. P. et al. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 22, 5323–5335 (2003)
Moberg, K., Starz, M. A. & Lees, J. A. E2F4 switches from p130 to p107 and pRB in response to cell cycle reentry. Mol. Cell. Biol. 16, 1436–1449 (1996)
Viatour, P. et al. Hematopoietic stem cell quiescence is maintained by compound contributions of the retinoblastoma gene family. Cell Stem Cell 3, 416–428 (2008)
Brelje, T. C., Parsons, J. A. & Sorenson, R. L. Regulation of islet β-cell proliferation by prolactin in rat islets. Diabetes 43, 263–273 (1994)
Scherping, S. C., Jr et al. Effect of growth factors on the proliferation of ligament fibroblasts from skeletally mature rabbits. Connect. Tissue Res. 36, 1–8 (1997)
Pallante, B. A. et al. Bone marrow Oct3/4+ cells differentiate into cardiac myocytes via age-dependent paracrine mechanisms. Circ. Res. 100, e1–e11 (2007)
LeBras, S., Czernichow, P. & Scharfmann, R. A search for tyrosine kinase receptors expressed in the rat embryonic pancreas. Diabetologia 41, 1474–1481 (1998)
Fujii, S. et al. MEK–ERK pathway regulates EZH2 overexpression in association with aggressive breast cancer subtypes. Oncogene 10.1038/onc.2011.118 (18 April 2011)
Gupta, R. K. et al. Expansion of adult β-cell mass in response to increased metabolic demand is dependent on HNF-4α. Genes Dev. 21, 756–769 (2007)
Miettinen, P. J. et al. Downregulation of EGF receptor signaling in pancreatic islets causes diabetes due to impaired postnatal β-cell growth. Diabetes 55, 3299–3308 (2006)
Butler, A. E. et al. Adaptive changes in pancreatic β-cell fractional area and β-cell turnover in human pregnancy. Diabetologia 53, 2167–2176 (2010)
Rieck, S. & Kaestner, K. H. Expansion of β-cell mass in response to pregnancy. Trends Endocrinol. Metab. 21, 151–158 (2010)
Ebert, M. et al. Induction of platelet-derived growth factor A and B chains and over-expression of their receptors in human pancreatic cancer. Int. J. Cancer 62, 529–535 (1995)
Nyblom, H. K. et al. Apoptotic, regenerative, and immune-related signaling in human islets from type 2 diabetes individuals. J. Proteome Res. 8, 5650–5656 (2009)
Tallquist, M. D. & Soriano, P. Cell autonomous requirement for PDGFRα in populations of cranial and cardiac neural crest cells. Development 130, 507–518 (2003)
Herrera, P. L. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 127, 2317–2322 (2000)
Hara, M. et al. Transgenic mice with green fluorescent protein-labeled pancreatic β-cells. Am. J. Physiol. Endocrinol. Metab. 284, E177–E183 (2003)
Sugiyama, T. et al. Conserved markers of fetal pancreatic epithelium permit prospective isolation of islet progenitor cells by FACS. Proc. Natl Acad. Sci. USA 104, 175–180 (2007)
Acknowledgements
We thank A. Bhushan, A. Stewart, A. Powers, P. Beachy, M. White, X. Chen and X. Li for helpful discussions and advice, A. Tarakhovsky, P. Herrera and M. Hara for mice, A. Powers, A. Thompson and S. Bryant for human islet sample procurement, and members of the S.K.K. laboratory for comments on the manuscript. H.C. was supported by the NIH Ruth L. Kirschstein NRSA/Stanford Regenerative Medicine Training Program. J.S. was supported by NIH-NCI RO1 CA114102. H.S. was supported by the Stem Cell Network NRW and Deutsche Krebshilfe. Work in the S.K.K. laboratory was supported by a gift from the Dewey family fund, and grants from the Juvenile Diabetes Research Foundation, Snyder Foundation, Stinehart Foundation, the NIH Beta Cell Biology Consortium (UO1 DK89532 to S.K.K. and UO1 DK89572 to A. Powers) and by the Howard Hughes Medical Institute (HHMI). S.K.K. is an Investigator of the HHMI.
Author information
Authors and Affiliations
Contributions
H.C., X G., Y.L. and J.W. performed experiments. H.C., S.E.W., J.S. and H.S. generated mice. R.B. isolated human islets. H.C. and S.K.K. conceived the project, generated hypotheses, analysed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains Supplementary Text, Supplementary Tables 1-3 and Supplementary Figures 1-13 with legends. (PDF 17810 kb)
Rights and permissions
About this article
Cite this article
Chen, H., Gu, X., Liu, Y. et al. PDGF signalling controls age-dependent proliferation in pancreatic β-cells. Nature 478, 349–355 (2011). https://doi.org/10.1038/nature10502
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10502
This article is cited by
-
Checkpoint kinase 2 controls insulin secretion and glucose homeostasis
Nature Chemical Biology (2023)
-
Genetic and pharmacologic inhibition of ALDH1A3 as a treatment of β-cell failure
Nature Communications (2023)
-
Ontology of the apelinergic system in mouse pancreas during pregnancy and relationship with β-cell mass
Scientific Reports (2021)
-
Coordinated interactions between endothelial cells and macrophages in the islet microenvironment promote β cell regeneration
npj Regenerative Medicine (2021)
-
Characterisation of Ppy-lineage cells clarifies the functional heterogeneity of pancreatic beta cells in mice
Diabetologia (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.