Abstract
The past decade has seen fundamental advances in our understanding of the ageing process and raised optimism that interventions to slow ageing may be on the horizon. Studies of budding yeast have made immense contributions to this progress. Yeast longevity factors have now been shown to modulate ageing in invertebrate and mammalian models, and studies of yeast have resulted in some of the best candidates for anti-ageing drugs currently in development. The first interventions to slow human ageing may spring from the humble yeast.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
01 April 2010
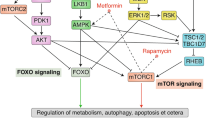
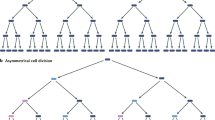
Nature 464, 513–519 (2010) Figure 3 of this Review contained a minor error. The correct figure is shown below.
References
Steinkraus, K. A., Kaeberlein, M. & Kennedy, B. K. Replicative aging in yeast: the means to the end. Annu. Rev. Cell Dev. Biol. 24, 29–54 (2008).
Fabrizio, P. & Longo, V. D. The chronological life span of Saccharomyces cerevisiae . Methods Mol. Biol. 371, 89–95 (2007).
Kennedy, B. K., Austriaco, N. R. Jr & Guarente, L. Daughter cells of Saccharomyces cerevisiae from old mothers display a reduced life span. J. Cell Biol. 127, 1985–1993 (1994).
Egilmez, N. K. & Jazwinski, S. M. Evidence for the involvement of a cytoplasmic factor in the aging of the yeast Saccharomyces cerevisiae . J. Bacteriol. 171, 37–42 (1989).
Ashrafi, K., Sinclair, D., Gordon, J. I. & Guarente, L. Passage through stationary phase advances replicative aging in Saccharomyces cerevisiae . Proc. Natl Acad. Sci. USA 96, 9100–9105 (1999).
Burtner, C. R., Murakami, C. J., Kennedy, B. K. & Kaeberlein, M. A molecular mechanism of chronological aging in yeast. Cell Cycle 8, 1256–1270 (2009). In this paper, acetic acid toxicity was shown to be the primary cause of chronological senescence under standard growth conditions.
Burhans, W. C. & Weinberger, M. Acetic acid effects on aging in budding yeast: are they relevant to aging in higher eukaryotes? Cell Cycle 8, 2300–2302 (2009).
Rockenfeller, P. & Madeo, F. Apoptotic death of ageing yeast. Exp. Gerontol. 43, 876–881 (2008).
Fabrizio, P. et al. SOD2 functions downstream of Sch9 to extend longevity in yeast. Genetics 163, 35–46 (2003).
Herker, E. et al. Chronological aging leads to apoptosis in yeast. J. Cell Biol. 164, 501–507 (2004).
Fabrizio, P. & Longo, V. D. Chronological aging-induced apoptosis in yeast. Biochim. Biophys. Acta 1783, 1280–1285 (2008).
Fabrizio, P. et al. Sir2 blocks extreme life-span extension. Cell 123, 655–667 (2005).
Powers, R. W. III, Kaeberlein, M., Caldwell, S. D., Kennedy, B. K. & Fields, S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 20, 174–184 (2006).
Harrison, D. E. et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009).
Fabrizio, P., Pozza, F., Pletcher, S. D., Gendron, C. M. & Longo, V. D. Regulation of longevity and stress resistance by Sch9 in yeast. Science 292, 288–290 (2001).
Kaeberlein, M. & Kapahi, P. Aging is RSKy business. Science 326, 55–56 (2009).
Ganley, A. R., Ide, S., Saka, K. & Kobayashi, T. The effect of replication initiation on gene amplification in the rDNA and its relationship to aging. Mol. Cell 35, 683–693 (2009).
Shcheprova, Z., Baldi, S., Frei, S. B., Gonnet, G. & Barral, Y. A mechanism for asymmetric segregation of age during yeast budding. Nature 454, 728–734 (2008). This study describes the existence of a septin-dependent diffusion barrier required for asymmetrical inheritance of nuclear pores and extrachromosomal rDNA circles by the mother cell during division.
Aguilaniu, H., Gustafsson, L., Rigoulet, M. & Nystrom, T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science 299, 1751–1753 (2003). This study showed that oxidatively damaged cytoplasmic proteins are asymmetrically segregated to the mother cell during ageing in a Sir2-dependent manner.
Erjavec, N. & Nystrom, T. Sir2p-dependent protein segregation gives rise to a superior reactive oxygen species management in the progeny of Saccharomyces cerevisiae . Proc. Natl Acad. Sci. USA 104, 10877–10881 (2007).
Lai, C. Y., Jaruga, E., Borghouts, C. & Jazwinski, S. M. A mutation in the ATP2 gene abrogates the age asymmetry between mother and daughter cells of the yeast Saccharomyces cerevisiae . Genetics 162, 73–87 (2002).
Kirchman, P. A., Kim, S., Lai, C. Y. & Jazwinski, S. M. Interorganelle signaling is a determinant of longevity in Saccharomyces cerevisiae . Genetics 152, 179–190 (1999).
Veatch, J. R., McMurray, M. A., Nelson, Z. W. & Gottschling, D. E. Mitochondrial dysfunction leads to nuclear genome instability via an iron-sulfur cluster defect. Cell 137, 1247–1258 (2009).
McMurray, M. A. & Gottschling, D. E. An age-induced switch to a hyper-recombinational state. Science 301, 1908–1911 (2003).
Kennedy, B. K., Steffen, K. K. & Kaeberlein, M. Ruminations on dietary restriction and aging. Cell. Mol. Life Sci. 64, 1323–1328 (2007).
Kaeberlein, M., Kirkland, K. T., Fields, S. & Kennedy, B. K. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2, e296 (2004).
Lin, S. J., Defossez, P. A. & Guarente, L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae . Science 289, 2126–2128 (2000).
Murakami, C. J., Burtner, C. R., Kennedy, B. K. & Kaeberlein, M. A method for high-throughput quantitative analysis of yeast chronological life span. J. Gerontol. A 63, 113–121 (2008).
Smith, D. L. Jr, McClure, J. M., Matecic, M. & Smith, J. S. Calorie restriction extends the chronological lifespan of Saccharomyces cerevisiae independently of the sirtuins. Aging Cell 6, 649–662 (2007).
Jiang, J. C., Jaruga, E., Repnevskaya, M. V. & Jazwinski, S. M. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J. 14, 2135–2137 (2000).
Alvers, A. L. et al. Autophagy and amino acid homeostasis are required for chronological longevity in Saccharomyces cerevisiae . Aging Cell 8, 353–369 (2009).
Finkel, T., Deng, C. X. & Mostoslavsky, R. Recent progress in the biology and physiology of sirtuins. Nature 460, 587–591 (2009).
Kaeberlein, M., McVey, M. & Guarente, L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 13, 2570–2580 (1999).
Kennedy, B. K. et al. Redistribution of silencing proteins from telomeres to the nucleolus is associated with extension of life span in S. cerevisiae . Cell 89, 381–391 (1997).
Kennedy, B. K. & Kaeberlein, M. Hot topics in aging research: protein translation, 2009. Aging Cell 8, 617–623 (2009).
Dang, W. et al. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature 459, 802–807 (2009). In this paper, a specific chromatin modification, H4K16 acetylation, was shown to be an important Sir2-regulated function in determining RLS.
Blackburn, E. H., Greider, C. W. & Szostak, J. W. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nature Med. 12, 1133–1138 (2006).
Shawi, M. & Autexier, C. Telomerase, senescence and ageing. Mech. Ageing Dev. 129, 3–10 (2008).
Austriaco, N. R. Jr & Guarente, L. P. Changes of telomere length cause reciprocal changes in the lifespan of mother cells in Saccharomyces cerevisiae . Proc. Natl Acad. Sci. USA 94, 9768–9772 (1997).
Guarente, L. Calorie restriction and SIR2 genes — towards a mechanism. Mech. Ageing Dev. 126, 923–928 (2005).
Kaeberlein, M. & Powers, R. W. III . Sir2 and calorie restriction in yeast: a skeptical perspective. Ageing Res. Rev. 6, 128–140 (2007).
Wullschleger, S., Loewith, R. & Hall, M. N. TOR signaling in growth and metabolism. Cell 124, 471–484 (2006).
Stanfel, M. N., Shamieh, L. S., Kaeberlein, M. & Kennedy, B. K. The TOR pathway comes of age. Biochim. Biophys. Acta 1790, 1067–1074 (2009).
Smith, E. D. et al. Quantitative evidence for conserved longevity pathways between divergent eukaryotic species. Genome Res. 18, 564–570 (2008). This study demonstrated that genetic control of longevity has been conserved between yeast and nematodes and identified 25 homologue pairs that modulate longevity in both species.
Kaeberlein, M. et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 310, 1193–1196 (2005).
Selman, C. et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian lifespan. Science 326, 140–144 (2009).
Chiocchetti, A. et al. Ribosomal proteins Rpl10 and Rps6 are potent regulators of yeast replicative life span. Exp. Gerontol. 42, 275–286 (2007).
Managbanag, J. R. et al. Shortest-path network analysis is a useful approach toward identifying genetic determinants of longevity. PLoS ONE 3, e3802 (2008).
Steffen, K. K. et al. Yeast life span extension by depletion of 60S ribosomal subunits is mediated by Gcn4. Cell 133, 292–302 (2008). This study defined the function of the large ribosomal subunit as a key determinant of yeast ageing and identified the transcription factor Gcn4 as a translationally regulated longevity factor.
Zid, B. M. et al. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila . Cell 139, 149–160 (2009).
Wang, X., Zuo, X., Kucejova, B. & Chen, X. J. Reduced cytosolic protein synthesis suppresses mitochondrial degeneration. Nature Cell Biol. 10, 1090–1097 (2008).
Riesen, M. & Morgan, A. Calorie restriction reduces rDNA recombination independently of rDNA silencing. Aging Cell 8, 624–632.
Medvedik, O., Lamming, D. W., Kim, K. D. & Sinclair, D. A. MSN2 and MSN4 link calorie restriction and TOR to sirtuin-mediated lifespan extension in Saccharomyces cerevisiae . PLoS Biol. 5, e261 (2007).
Beck, T. & Hall, M. N. The TOR signalling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature 402, 689–692 (1999).
Anderson, R. M., Bitterman, K. J., Wood, J. G., Medvedik, O. & Sinclair, D. A. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae . Nature 423, 181–185 (2003).
Smith, D. L. et al. Calorie restriction effects on silencing and recombination at the yeast rDNA. Aging Cell 8, 633–642 (2009).
Bonawitz, N. D., Chatenay- Lapointe, M., Pan, Y. & Shadel, G. S. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell. Metab. 5, 265–277 (2007).
Alvers, A. L. et al. Autophagy is required for extension of yeast chronological life span by rapamycin. Autophagy 5, 847–849 (2009).
Wei, M. et al. Tor1/Sch9-regulated carbon source substitution is as effective as calorie restriction in life span extension. PLoS Genet. 5, e1000467 (2009).
Roux, A. E. et al. Pro-aging effects of glucose signaling through a G protein-coupled glucose receptor in fission yeast. PLoS Genet. 5, e1000408 (2009).
Roux, A. E., Quissac, A., Chartrand, P., Ferbeyre, G. & Rokeach, L. A. Regulation of chronological aging in Schizosaccharomyces pombe by the protein kinases Pka1 and Sck2. Aging Cell 5, 345–357 (2006).
Barker, M. G. & Walmsley, R. M. Replicative ageing in the fission yeast Schizosaccharomyces pombe . Yeast 15, 1511–1518 (1999).
Erjavec, N., Cvijovic, M., Klipp, E. & Nystrom, T. Selective benefits of damage partitioning in unicellular systems and its effects on aging. Proc. Natl Acad. Sci. USA 105, 18764–18769 (2008).
Oliveira, G. A., Tahara, E. B., Gombert, A. K., Barros, M. H. & Kowaltowski, A. J. Increased aerobic metabolism is essential for the beneficial effects of caloric restriction on yeast life span. J. Bioenerg. Biomembr. 40, 381–388 (2008).
Kaeberlein, M. Resveratrol and rapamycin: are they anti-aging drugs? BioEssays 32, 96–99 (2010).
Lorenz, D. R., Cantor, C. R. & Collins, J. J. A network biology approach to aging in yeast. Proc. Natl Acad. Sci. USA 106, 1145–1150 (2009).
Lindstrom, D. L. & Gottschling, D. E. The mother enrichment program: a genetic system for facile replicative life span analysis in Saccharomyces cerevisiae . Genetics 183, 413–422 (2009).
Qin, H., Lu, M. & Goldfarb, D. S. Genomic instability is associated with natural life span variation in Saccharomyces cerevisiae . PLoS ONE 3, e2670 (2008).
Lamming, D. W. et al. HST2 mediates SIR2-independent life-span extension by calorie restriction. Science 309, 1861–1864 (2005).
Kaeberlein, M. et al. Comment on 'HST2 mediates SIR2-independent life-span extension by calorie restriction'. Science 312, 1312 (2006).
Tsuchiya, M. et al. Sirtuin-independent effects of nicotinamide on lifespan extension from calorie restriction in yeast. Aging Cell 5, 505–514 (2006).
Lu, S. P. & Lin, S. J. Regulation of yeast sirtuins by NAD+ metabolism and calorie restriction. Biochim. Biophys. Acta doi:10.1016/j.bbapap.2009.09.030 (in the press).
Kaeberlein, M. et al. Increased life span due to calorie restriction in respiratory-deficient yeast. PLoS Genet. 1, e69 (2005).
Howitz, K. T. et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425, 191–196 (2003).
Wood, J. G. et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430, 686–689 (2004).
Valenzano, D. R. et al. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol. 16, 296–300 (2006).
Borra, M. T., Smith, B. C. & Denu, J. M. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 280, 17187–17195 (2005).
Kaeberlein, M. et al. Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem. 280, 17038–17045 (2005).
Bass, T. M., Weinkove, D., Houthoofd, K., Gems, D. & Partridge, L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans . Mech. Ageing Dev. 128, 546–552 (2007).
Beher, D. et al. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem. Biol. Drug Des. 74, 619–624 (2009).
Baur, J. A. et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444, 337–342 (2006).
Lagouge, M. et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127, 1109–1122 (2006).
Barger, J. L. et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE 3, e2264 (2008).
Pearson, K. J. et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell. Metab. 8, 157–168 (2008).
Kaeberlein, M. Spermidine surprise for a long life. Nature Cell Biol. 11, 1277–1278 (2009).
Eisenberg, T. et al. Induction of autophagy by spermidine promotes longevity. Nature Cell Biol. 11, 1277–1278 (2009). This study identified spermidine as a compound that increases lifespan in yeast, nematodes and flies.
Acknowledgements
Studies related to this topic in the Kaeberlein laboratory have been supported by US National Institutes of Health (NIH) grant R21AG031965, a Pilot Project grant from the University of Washington Nathan Shock Center of Excellence in the Basic Biology of Aging (NIH grant P30AG013280) and a New Scholar in Aging Award from the Ellison Medical Foundation.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
M.K. has been issued a patent issued for the identification of ageing genes through large-scale analysis (US patent 7,622,271).
Additional information
Reprints and permissions information is available at http://www.nature.com/reprints. The author declares competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/nature. Correspondence should be addressed to the author (kaeber@u.washington.edu).
Rights and permissions
About this article
Cite this article
Kaeberlein, M. Lessons on longevity from budding yeast. Nature 464, 513–519 (2010). https://doi.org/10.1038/nature08981
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08981
This article is cited by
-
Artificial Hsp104-mediated systems for re-localizing protein aggregates
Nature Communications (2023)
-
Physiological and genetic regulation of anhydrobiosis in yeast cells
Archives of Microbiology (2023)
-
The nexus between peroxisome abundance and chronological ageing in Saccharomyces cerevisiae
Biogerontology (2023)
-
Antagonistic effects of mitochondrial matrix and intermembrane space proteases on yeast aging
BMC Biology (2022)
-
The role of NAD and NAD precursors on longevity and lifespan modulation in the budding yeast, Saccharomyces cerevisiae
Biogerontology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.