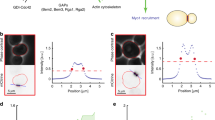
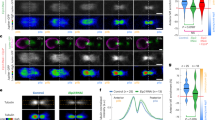
Abstract
Cells normally grow to a certain size before they enter mitosis and divide. Entry into mitosis depends on the activity of Cdk1, which is inhibited by the Wee1 kinase and activated by the Cdc25 phosphatase1. However, how cells sense their size for mitotic commitment remains unknown. Here we show that an intracellular gradient of the dual-specificity tyrosine-phosphorylation regulated kinase (DYRK) Pom1, which emanates from the ends of rod-shaped Schizosaccharomyces pombe cells, serves to measure cell length and control mitotic entry. Pom1 provides positional information both for polarized growth and to inhibit cell division at cell ends2,3,4,5. We discovered that Pom1 is also a dose-dependent G2–M inhibitor. Genetic analyses indicate that Pom1 negatively regulates Cdr1 and Cdr2, two previously described Wee1 inhibitors of the SAD kinase family6,7,8,9,10. This inhibition may be direct, because in vivo and in vitro evidence suggest that Pom1 phosphorylates Cdr2. Whereas Cdr1 and Cdr2 localize to a medial cortical region, Pom1 forms concentration gradients from cell tips that overlap with Cdr1 and Cdr2 in short cells, but not in long cells. Disturbing these Pom1 gradients leads to Cdr2 phosphorylation and imposes a G2 delay. In short cells, Pom1 prevents precocious M-phase entry, suggesting that the higher medial Pom1 levels inhibit Cdr2 and promote a G2 delay. Thus, gradients of Pom1 from cell ends provide a measure of cell length to regulate M-phase entry.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Nurse, P. Universal control mechanism regulating onset of M-phase. Nature 344, 503–508 (1990)
Bähler, J. & Pringle, J. R. Pom1p, a fission yeast protein kinase that provides positional information for both polarized growth and cytokinesis. Genes Dev. 12, 1356–1370 (1998)
Celton-Morizur, S., Racine, V., Sibarita, J. B. & Paoletti, A. Pom1 kinase links division plane position to cell polarity by regulating Mid1p cortical distribution. J. Cell Sci. 119, 4710–4718 (2006)
Huang, Y., Chew, T. G., Ge, W. & Balasubramanian, M. K. Polarity determinants Tea1p, Tea4p, and Pom1p inhibit division-septum assembly at cell ends in fission yeast. Dev. Cell 12, 987–996 (2007)
Padte, N. N., Martin, S. G., Howard, M. & Chang, F. The cell-end factor Pom1p inhibits Mid1p in specification of the cell division plane in fission yeast. Curr. Biol. 16, 2480–2487 (2006)
Coleman, T. R., Tang, Z. & Dunphy, W. G. Negative regulation of the wee1 protein kinase by direct action of the nim1/cdr1 mitotic inducer. Cell 72, 919–929 (1993)
Parker, L. L., Walter, S. A., Young, P. G. & Piwnica-Worms, H. Phosphorylation and inactivation of the mitotic inhibitor Wee1 by the nim1/cdr1 kinase. Nature 363, 736–738 (1993)
Wu, L. & Russell, P. Nim1 kinase promotes mitosis by inactivating Wee1 tyrosine kinase. Nature 363, 738–741 (1993)
Breeding, C. S. et al. The cdr2+ gene encodes a regulator of G2/M progression and cytokinesis in Schizosaccharomyces pombe . Mol. Biol. Cell 9, 3399–3415 (1998)
Kanoh, J. & Russell, P. The protein kinase Cdr2, related to Nim1/Cdr1 mitotic inducer, regulates the onset of mitosis in fission yeast. Mol. Biol. Cell 9, 3321–3334 (1998)
Petersen, J. & Hagan, I. M. Polo kinase links the stress pathway to cell cycle control and tip growth in fission yeast. Nature 435, 507–512 (2005)
Shiozaki, K. & Russell, P. Cell-cycle control linked to extracellular environment by MAP kinase pathway in fission yeast. Nature 378, 739–743 (1995)
Wu, L. & Russell, P. Nif1, a novel mitotic inhibitor in Schizosaccharomyces pombe . EMBO J. 16, 1342–1350 (1997)
Bähler, J. & Nurse, P. Fission yeast Pom1p kinase activity is cell cycle regulated and essential for cellular symmetry during growth and division. EMBO J. 20, 1064–1073 (2001)
Lundgren, K. et al. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell 64, 1111–1122 (1991)
Parker, L. L., Atherton-Fessler, S. & Piwnica-Worms, H. p107wee1 is a dual-specificity kinase that phosphorylates p34cdc2 on tyrosine 15. Proc. Natl Acad. Sci. USA 89, 2917–2921 (1992)
Morrell, J. L., Nichols, C. B. & Gould, K. L. The GIN4 family kinase, Cdr2p, acts independently of septins in fission yeast. J. Cell Sci. 117, 5293–5302 (2004)
Tatebe, H., Shimada, K., Uzawa, S., Morigasaki, S. & Shiozaki, K. Wsh3/Tea4 is a novel cell-end factor essential for bipolar distribution of Tea1 and protects cell polarity under environmental stress in S. pombe . Curr. Biol. 15, 1006–1015 (2005)
Martin, S. G., McDonald, W. H., Yates, J. R. & Chang, F. Tea4p links microtubule plus ends with the formin for3p in the establishment of cell polarity. Dev. Cell 8, 479–491 (2005)
Mata, J. & Nurse, P. tea1 and the microtubular cytoskeleton are important for generating global spatial order within the fission yeast cell. Cell 89, 939–949 (1997)
Russell, P. & Nurse, P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell 49, 559–567 (1987)
Tapon, N., Moberg, K. H. & Hariharan, I. K. The coupling of cell growth to the cell cycle. Curr. Opin. Cell Biol. 13, 731–737 (2001)
Kalab, P., Pralle, A., Isacoff, E. Y., Heald, R. & Weis, K. Analysis of a RanGTP-regulated gradient in mitotic somatic cells. Nature 440, 697–701 (2006)
Fuller, B. G. et al. Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient. Nature 453, 1132–1136 (2008)
Souza, G. M., Lu, S. & Kuspa, A. YakA, a protein kinase required for the transition from growth to development in Dictyostelium . Development 125, 2291–2302 (1998)
Pellettieri, J., Reinke, V., Kim, S. K. & Seydoux, G. Coordinate activation of maternal protein degradation during the egg-to-embryo transition in C. elegans . Dev. Cell 5, 451–462 (2003)
Mercer, S. E. & Friedman, E. Mirk/Dyrk1B: a multifunctional dual-specificity kinase involved in growth arrest, differentiation, and cell survival. Cell Biochem. Biophys. 45, 303–315 (2006)
Kishi, M., Pan, Y. A., Crump, J. G. & Sanes, J. R. Mammalian SAD kinases are required for neuronal polarization. Science 307, 929–932 (2005)
Lew, D. J. The morphogenesis checkpoint: how yeast cells watch their figures. Curr. Opin. Cell Biol. 15, 648–653 (2003)
Bähler, J. et al. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe . Yeast 14, 943–951 (1998)
Snaith, H. A., Samejima, I. & Sawin, K. E. Multistep and multimode cortical anchoring of tea1p at cell tips in fission yeast. EMBO J. 24, 3690–3699 (2005)
Wu, J. Q. et al. Assembly of the cytokinetic contractile ring from a broad band of nodes in fission yeast. J. Cell Biol. 174, 391–402 (2006)
Acknowledgements
We wish to thank J. Bähler, F. Chang, P. Cruz, K. Gull, I. Hagan, P. Russell, K. Shiozaki and V. Simanis for strains and reagents. We thank R. Benton, F. Chang, C. Fankhauser, N. Hernandez and F. Bendezu for critical reading of the manuscript. We also wish to thank J. Moseley and P. Nurse for open discussion before publication. Research in the laboratory of S.G.M. is supported by a Swiss National Science Foundation Professorship grant (PP00A–114936) and a Human Frontiers Science Program Career Development Award (CDA0016/2008).
Author Contributions S.G.M. conceived the project, and designed and analysed the experiments. S.G.M. and M.B.G. performed the experiments. The manuscript was written by S.G.M. with input from M.B.G.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-9 with Legends and Supplementary Table 1. (PDF 6051 kb)
Rights and permissions
About this article
Cite this article
Martin, S., Berthelot-Grosjean, M. Polar gradients of the DYRK-family kinase Pom1 couple cell length with the cell cycle. Nature 459, 852–856 (2009). https://doi.org/10.1038/nature08054
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08054
This article is cited by
-
Control of protein-based pattern formation via guiding cues
Nature Reviews Physics (2022)
-
Functional interaction between Cdc42 and the stress MAPK signaling pathway during the regulation of fission yeast polarized growth
International Microbiology (2020)
-
Cell size sensing—a one-dimensional solution for a three-dimensional problem?
BMC Biology (2019)
-
Genome-wide association analysis of stalk biomass and anatomical traits in maize
BMC Plant Biology (2019)
-
Fission yeast type 2 node proteins Blt1p and Gef2p cooperate to ensure timely completion of cytokinesis
BMC Molecular and Cell Biology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.