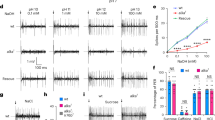
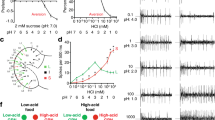
Abstract
There are five known taste modalities in humans: sweet, bitter, sour, salty and umami (the taste of monosodium glutamate). Although the fruitfly Drosophila melanogaster tastes sugars, salts and noxious chemicals, the nature and number of taste modalities in this organism is not clear. Previous studies have identified one taste cell population marked by the gustatory receptor gene Gr5a that detects sugars, and a second population marked by Gr66a that detects bitter compounds1,2,3,4. Here we identify a novel taste modality in this insect: the taste of carbonated water. We use a combination of anatomical, calcium imaging and behavioural approaches to identify a population of taste neurons that detects CO2 and mediates taste acceptance behaviour. The taste of carbonation may allow Drosophila to detect and obtain nutrients from growing microorganisms. Whereas CO2 detection by the olfactory system mediates avoidance5, CO2 detection by the gustatory system mediates acceptance behaviour, demonstrating that the context of CO2 determines appropriate behaviour. This work opens up the possibility that the taste of carbonation may also exist in other organisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chyb, S., Dahanukar, A., Wickens, A. & Carlson, J. R. Drosophila Gr5a encodes a taste receptor tuned to trehalose. Proc. Natl Acad. Sci. USA 100 (suppl. 2). 14526–14530 (2003)
Thorne, N., Chromey, C., Bray, S. & Amrein, H. Taste perception and coding in Drosophila.. Curr. Biol. 14, 1065–1079 (2004)
Wang, Z., Singhvi, A., Kong, P. & Scott, K. Taste representations in the Drosophila brain. Cell 117, 981–991 (2004)
Marella, S. et al. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron 49, 285–295 (2006)
Suh, G. S. et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature 431, 854–859 (2004)
Stocker, R. F. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 275, 3–26 (1994)
Baines, R. A., Uhler, J. P., Thompson, A., Sweeney, S. T. & Bate, M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J. Neurosci. 21, 1523–1531 (2001)
McGuire, S. E., Mao, Z. & Davis, R. L. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci. STKE 2004, pl6 (2004)
Jordt, S. E., Tominaga, M. & Julius, D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc. Natl Acad. Sci. USA 97, 8134–8139 (2000)
Jones, W. D., Cayirlioglu, P., Kadow, I. G. & Vosshall, L. B. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature 445, 86–90 (2007)
Kwon, J. Y., Dahanukar, A., Weiss, L. A. & Carlson, J. R. The molecular basis of CO2 reception in Drosophila. Proc. Natl Acad. Sci. USA 104, 3574–3578 (2007)
Scott, K. et al. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell 104, 661–673 (2001)
Clyne, P. J., Warr, C. G. & Carlson, J. R. Candidate taste receptors in Drosophila. Science 287, 1830–1834 (2000)
Dunipace, L., Meister, S., McNealy, C. & Amrein, H. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr. Biol. 11, 822–835 (2001)
Robertson, H. M., Warr, C. G. & Carlson, J. R. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 100 (Suppl 2). 14537–14542 (2003)
Coates, E. L. Olfactory CO2 chemoreceptors. Respir. Physiol. 129, 219–229 (2001)
Gray, J. M. et al. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature 430, 317–322 (2004)
Verma, A., Hirsch, D. J., Glatt, C. E., Ronnett, G. V. & Snyder, S. H. Carbon monoxide: a putative neural messenger. Science 259, 381–384 (1993)
Wingrove, J. A. & O’Farrell, P. H. Nitric oxide contributes to behavioral, cellular, and developmental responses to low oxygen in Drosophila.. Cell 98, 105–114 (1999)
Faucher, C., Forstreuter, M., Hilker, M. & de Bruyne, M. Behavioral responses of Drosophila to biogenic levels of carbon dioxide depend on life-stage, sex and olfactory context. J. Exp. Biol. 209, 2739–2748 (2006)
Inoshita, T. & Tanimura, T. Cellular identification of water gustatory receptor neurons and their central projection pattern in Drosophila. Proc. Natl Acad. Sci. USA 103, 1094–1099 (2006)
Hummel, T., Krukkert, K., Roos, J., Davis, G. & Klambt, C. Drosophila Futsch/22C10 is a MAP1B-like protein required for dendritic and axonal development. Neuron 26, 357–370 (2000)
Acknowledgements
We thank U. Heberlein and her laboratory for generating and providing the Gal4 enhancer trap library containing the E409-Gal4 transgenic flies; Z. Wang for generation of the Gr5a-GFP-IRES-GFP-IRES-GFP transgenic flies; S. Asgarian for technical assistance in the anatomy screen; and G. Agarwaal for assistance with Matlab. We also thank L. Vosshall, C. Zuker and members of the Scott laboratory for providing comments on the manuscript. This work was supported by a grant from the NIH (NIDCD), a Burroughs Wellcome Fund Career Award, a McKnight Scholar Award and a John Merck Award to K.S.
Author Contributions W.F. performed the majority of G-CaMP imaging experiments, developed the behavioural assay, performed behavioural experiments and co-wrote the manuscript. P.K. performed the anatomy screen leading to the identification of E409-Gal4. S.M. performed the G-CaMP imaging experiments of capsaicin-induced responses in E409-Gal4, UAS-GCaMP, UAS-VR1E600K flies, sequenced the Gal4 insertion site in the E409-Gal4 flies and participated in initial characterization of the E409 neurons. K.S. assisted P.K. in the anatomy screen and W.F. in the G-CaMP imaging and behavioural studies, co-wrote the manuscript and supervised the project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figure 1 with Legend. The Supplemental Figure provides a schematic of fly taste anatomy and the expression pattern of E409 in the fly brain. (PDF 161 kb)
Rights and permissions
About this article
Cite this article
Fischler, W., Kong, P., Marella, S. et al. The detection of carbonation by the Drosophila gustatory system. Nature 448, 1054–1057 (2007). https://doi.org/10.1038/nature06101
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature06101
This article is cited by
-
Molecular and cellular basis of acid taste sensation in Drosophila
Nature Communications (2021)
-
Recent advances in the genetic basis of taste detection in Drosophila
Cellular and Molecular Life Sciences (2020)
-
An expression atlas of variant ionotropic glutamate receptors identifies a molecular basis of carbonation sensing
Nature Communications (2018)
-
The taste response to ammonia in Drosophila
Scientific Reports (2017)
-
Nano-architecture of gustatory chemosensory bristles and trachea in Drosophila wings
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.