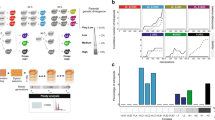
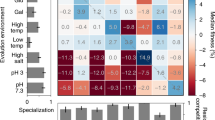
Abstract
Establishing the conditions that promote the evolution of reproductive isolation and speciation has long been a goal in evolutionary biology1,2,3. In ecological speciation, reproductive isolation between populations evolves as a by-product of divergent selection and the resulting environment-specific adaptations4,5,6. The leading genetic model of reproductive isolation predicts that hybrid inferiority is caused by antagonistic epistasis between incompatible alleles at interacting loci1,7. The fundamental link between divergent adaptation and reproductive isolation through genetic incompatibilities has been predicted1,4,5, but has not been directly demonstrated experimentally. Here we empirically tested key predictions of speciation theory by evolving the initial stages of speciation in experimental populations of the yeast Saccharomyces cerevisiae. After replicate populations adapted to two divergent environments, we consistently observed the evolution of two forms of postzygotic isolation in hybrids: reduced rate of mitotic reproduction and reduced efficiency of meiotic reproduction. This divergent selection resulted in greater reproductive isolation than parallel selection, as predicted by the ecological speciation theory. Our experimental system allowed controlled comparison of the relative importance of ecological and genetic isolation, and we demonstrated that hybrid inferiority can be ecological and/or genetic in basis. Overall, our results show that adaptation to divergent environments promotes the evolution of reproductive isolation through antagonistic epistasis, providing evidence of a plausible common avenue to speciation and adaptive radiation in nature.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Dobzhansky, T. Genetics and the Origin of Species (Columbia Univ. Press, New York, 1937)
Coyne, J. A. Genetics and speciation. Nature 355, 511–515 (1992)
Coyne, J. A. & Orr, H. A. Speciation (Sinauer, Sunderland, 2004)
Schluter, D. The Ecology of Adaptive Radiation (Oxford Univ. Press, Oxford, 2000)
Schluter, D. Ecology and the origin of species. Trends Ecol. Evol. 16, 372–380 (2001)
Rundle, H. D. & Nosil, P. Ecological speciation. Ecol. Lett. 8, 336–352 (2005)
Muller, H. J. Isolating mechanisms, evolution, and temperature. Biol. Symp. 6, 71–125 (1942)
McKinnon, J. S. et al. Evidence for ecology’s role in speciation. Nature 429, 294–298 (2004)
Funk, D. J., Nosil, P. & Etges, W. J. Ecological divergence exhibits consistently positive associations with reproductive isolation across disparate taxa. Proc. Natl Acad. Sci. USA 103, 3209–3213 (2006)
Orr, H. A. & Turelli, M. The evolution of postzygotic isolation: accumulating Dobzhansky–Muller incompatibilities. Evolution 55, 1085–1094 (2001)
Presgraves, D. C., Balagopalan, L., Abmayr, S. M. & Orr, H. A. Adaptive evolution drives divergence of a hybrid inviability gene between two species of Drosophila. Nature 423, 715–719 (2003)
Rundle, H. D. & Whitlock, M. C. A genetic interpretation of ecologically dependent isolation. Evolution 55, 198–201 (2001)
Demuth, J. P. & Wade, M. J. On the theoretical and empirical framework for studying genetic interactions within and among species. Am. Nat. 165, 524–536 (2005)
Anderson, J. B., Ricker, N. & Sirjusingh, C. Antagonism between two mechanisms of antifungal drug resistance. Eukaryot. Cell 5, 1243–1251 (2006)
Rieseberg, L. H. Chromosomal rearrangements and speciation. Trends Ecol. Evol. 16, 351–358 (2001)
Delneri, D. et al. Engineering evolution to study speciation in yeasts. Nature 422, 68–72 (2003)
Fischer, G., James, S. A., Roberts, I. N., Oliver, S. G. & Louis, E. J. Chromosomal evolution in Saccharomyces. Nature 405, 451–454 (2000)
Hunter, N., Chambers, S. R., Louis, E. J. & Borts, R. H. The mismatch repair system contributes to meiotic sterility in an interspecific yeast hybrid. EMBO J. 15, 1726–1733 (1996)
Greig, D., Travisano, M., Louis, E. J. & Borts, R. H. A role for the mismatch repair system during incipient speciation in Saccharomyces. J. Evol. Biol. 16, 429–437 (2003)
Chu, S. et al. The transcriptional program of sporulation in budding yeast. Science 282, 699–705 (1998)
Greig, D. A screen for recessive speciation genes expressed in the gametes of F1 hybrid yeast. PLoS Genet. 3, e21 (2007)
Greig, D., Borts, R. H., Louis, E. J. & Travisano, M. Epistasis and hybrid sterility in Saccharomyces. Proc. R. Soc. Lond. B 269, 1167–1171 (2002)
Rice, W. R. & Hostert, E. E. Laboratory experiments on speciation: what have we learned in 40 years? Evolution 47, 1637–1653 (1993)
Mooers, A. Ø., Rundle, H. D. & Whitlock, M. C. The effects of selection and bottlenecks on male mating success in peripheral isolates. Am. Nat. 153, 437–444 (1999)
Rundle, H. D. Divergent environments and population bottlenecks fail to generate premating isolation in Drosophila pseudoobscura. Evolution 57, 2557–2565 (2003)
Rundle, H. D., Chenoweth, S. F., Doughty, P. & Blows, M. W. Divergent selection and the evolution of signal traits and mating preferences. PLoS Biol. 3, e368 (2005)
Leu, J. Y. & Murray, A. W. Experimental evolution of mating discrimination in budding yeast. Curr. Biol. 16, 280–286 (2006)
de Oliveira, A. K. & Cordeiro, A. R. Adaptation of Drosophila willistoni experimental populations to extreme pH medium II. Development of incipient reproductive isolation. Heredity 44, 123–130 (1980)
Kohn, L. M. Mechanisms of fungal speciation. Annu. Rev. Phytopathol. 43, 279–308 (2005)
Anderson, J. B. et al. Mode of selection and experimental evolution of antifungal drug resistance in Saccharomyces cerevisiae. Genetics 163, 1287–1298 (2003)
Acknowledgements
This work was supported by Discovery grants to J.B.A. and L.M.K., and a Postdoctoral Fellowship to J.R.D., from the Natural Science and Engineering Research Council of Canada.
Author Contributions The research was conceived and planned by all authors. C.S. and J.R.D. performed the experiments, and J.R.D. analysed the data. J.R.D, J.B.A. and L.M.K. contributed to the writing of the manuscript, which was coordinated by J.R.D.
The full microarray data set has been deposited in the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) under accession series GSE6870.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file includes Supplementary Methods, Supplementary Material 1 (Evidence against alternative explanations for reduced meiotic efficiency of hybrids), Supplementary Material 2 (Genetic experiment to assess concomitant reductions in mitotic fitness and meiotic efficiency) Supplementary Material 3 (Microarray expression data), and Supplementary Material 4 (Validation of microarray expression data). (PDF 1199 kb)
Rights and permissions
About this article
Cite this article
Dettman, J., Sirjusingh, C., Kohn, L. et al. Incipient speciation by divergent adaptation and antagonistic epistasis in yeast. Nature 447, 585–588 (2007). https://doi.org/10.1038/nature05856
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature05856
This article is cited by
-
Experimental evolution of adaptive divergence under varying degrees of gene flow
Nature Ecology & Evolution (2021)
-
Interspecific hybridization as a driver of fungal evolution and adaptation
Nature Reviews Microbiology (2021)
-
Meiotic recombination in the offspring of Microbotryum hybrids and its impact on pathogenicity
BMC Evolutionary Biology (2020)
-
Ecology shapes epistasis in a genotype–phenotype–fitness map for stick insect colour
Nature Ecology & Evolution (2020)
-
Chance and necessity in the pleiotropic consequences of adaptation for budding yeast
Nature Ecology & Evolution (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.