Abstract
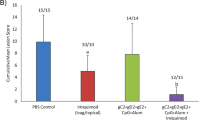
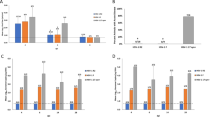
Herpes simplex virus 2 (HSV-2) infection causes significant morbidity1 and is an important cofactor for the transmission of HIV infection2. A microbicide to prevent sexual transmission of HSV-2 would contribute substantially to controlling the spread of HIV and other infections3,4. Because RNA interference (RNAi) provides effective antiviral defence in plants and other organisms, several studies have focused on harnessing RNAi to inhibit viral infection5. Here we show that vaginal instillation of small interfering RNAs (siRNAs) targeting HSV-2 protects mice from lethal infection. siRNAs mixed with lipid are efficiently taken up by epithelial and lamina propria cells and silence gene expression in the mouse vagina and ectocervix for at least nine days. Intravaginal application of siRNAs targeting the HSV-2 UL27 and UL29 genes (which encode an envelope glycoprotein and a DNA binding protein6, respectively) was well tolerated, did not induce interferon-responsive genes or cause inflammation, and protected mice when administered before and/or after lethal HSV-2 challenge. These results suggest that siRNAs are attractive candidates for the active component of a microbicide designed to prevent viral infection or transmission.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Whitley, R. J. in Field's Virology (eds Knipe, D. M. & Howley, P. M.) 2461–2510 (Lippincott, Williams and Wilkins, Philadelphia, 2001)
Wald, A. & Link, K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J. Infect. Dis. 185, 45–52 (2002)
Celum, C., Levine, R., Weaver, M. & Wald, A. Genital herpes and human immunodeficiency virus: double trouble. Bull. World Health Organ. 82, 447–453 (2004)
Pilcher, H. Starting to gel. Nature 430, 138–140 (2004)
Shankar, P., Manjunath, N. & Lieberman, J. The prospect of silencing disease using RNA interference. J. Am. Med. Assoc. 293, 1367–1373 (2005)
Roizman, B. & Knipe, D. M. in Field's Virology (eds Knipe, D. M. & Howley, P. M.) 2399–2440 (Lippincott, Williams and Wilkins, Philadelphia, 2001)
Hadjantonakis, A. K., Gertsenstein, M., Ikawa, M., Okabe, M. & Nagy, A. Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech. Dev. 76, 79–90 (1998)
Khvorova, A., Reynolds, A. & Jayasena, S. D. Functional siRNAs and miRNAs exhibit strand bias. Cell 115, 209–216 (2003)
Boden, D., Pusch, O., Lee, F., Tucker, L. & Ramratnam, B. Human immunodeficiency virus type 1 escape from RNA interference. J. Virol. 77, 11531–11535 (2003)
Gitlin, L., Stone, J. K. & Andino, R. Poliovirus escape from RNA interference: short interfering RNA-target recognition and implications for therapeutic approaches. J. Virol. 79, 1027–1035 (2005)
Wilson, J. A. & Richardson, C. D. Hepatitis C virus replicons escape RNA interference induced by a short interfering RNA directed against the NS5b coding region. J. Virol. 79, 7050–7058 (2005)
Sledz, C. A., Holko, M., de Veer, M. J., Silverman, R. H. & Williams, B. R. Activation of the interferon system by short-interfering RNAs. Nature Cell Biol. 5, 834–839 (2003)
Heidel, J. D., Hu, S., Liu, X. F., Triche, T. J. & Davis, M. E. Lack of interferon response in animals to naked siRNAs. Nature Biotechnol. 22, 1579–1582 (2004)
Hornung, V. et al. Sequence-specific potent induction of IFN-α by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nature Med. 11, 263–270 (2005)
Judge, A. D. et al. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nature Biotechnol. 23, 457–462 (2005)
Ge, Q. et al. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc. Natl Acad. Sci. USA 101, 8676–8681 (2004)
Tompkins, S. M., Lo, C. Y., Tumpey, T. M. & Epstein, S. L. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc. Natl Acad. Sci. USA 101, 8682–8686 (2004)
Bitko, V., Musiyenko, A., Shulyayeva, O. & Barik, S. Inhibition of respiratory viruses by nasally administered siRNA. Nature Med. 11, 50–55 (2005)
Zhang, W. et al. Inhibition of respiratory syncytial virus infection with intranasal siRNA nanoparticles targeting the viral NS1 gene. Nature Med. 11, 56–62 (2005)
Li, B. J. et al. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nature Med. 11, 944–951 (2005)
Manoharan, M. RNA interference and chemically modified small interfering RNAs. Curr. Opin. Chem. Biol. 8, 570–579 (2004)
Morrison, L. A., Da Costa, X. J. & Knipe, D. M. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology 243, 178–187 (1998)
Jones, C. A., Taylor, T. J. & Knipe, D. M. Biological properties of herpes simplex virus 2 replication-defective mutant strains in a murine nasal infection model. Virology 278, 137–150 (2000)
Gao, M. & Knipe, D. M. Genetic evidence for multiple nuclear functions of the herpes simplex virus ICP8 DNA-binding protein. J. Virol. 63, 5258–5267 (1989)
Spang, A. E., Godowski, P. J. & Knipe, D. M. Characterization of herpes simplex virus 2 temperature-sensitive mutants whose lesions map in or near the coding sequences for the major DNA-binding protein. J. Virol. 45, 332–342 (1983)
Novina, C. D. et al. siRNA-directed inhibition of HIV-1 infection. Nature Med. 8, 681–686 (2002)
Kaplan, E. L. & Meier, R. Non-parametric estimation from incomplete observation. J. Am. Stat. Assoc. 53, 457–481 (1958)
Mantel, N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. Rep. 50, 163–170 (1966)
Zeger, S. L. & Liang, K. Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42, 121–130 (1986)
Acknowledgements
We thank R. Colgrove, T. Taylor, E. Torres-Lopez, D. Brown and S. White for advice. This work was supported by grants from the NIH to D.M.K. and J.L., and by postdoctoral fellowships from the Harvard Center for AIDS Research and amfAR to D.P. and the Leukemia and Lymphoma Society to D.C.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Some of the authors have filed a patent application based on the work reported in this manuscript.
Rights and permissions
About this article
Cite this article
Palliser, D., Chowdhury, D., Wang, QY. et al. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature 439, 89–94 (2006). https://doi.org/10.1038/nature04263
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04263
This article is cited by
-
Innate immune regulations and various siRNA modalities
Drug Delivery and Translational Research (2023)
-
shRNA transgenic swine display resistance to infection with the foot-and-mouth disease virus
Scientific Reports (2021)
-
Vaginal siRNA delivery: overview on novel delivery approaches
Drug Delivery and Translational Research (2020)
-
Nuclear lactate dehydrogenase A senses ROS to produce α-hydroxybutyrate for HPV-induced cervical tumor growth
Nature Communications (2018)
-
Worldwide circulation of HSV-2 × HSV-1 recombinant strains
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.