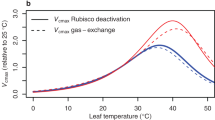
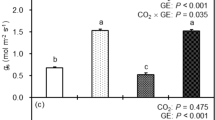
Abstract
Water stress substantially alters plant metabolism, decreasing plant growth and photosynthesis1,2,3,4 and profoundly affecting ecosystems and agriculture, and thus human societies5. There is controversy over the mechanisms by which stress decreases photosynthetic assimilation of CO2. Two principal effects are invoked2,4: restricted diffusion of CO2 into the leaf, caused by stomatal closure6,7,8, and inhibition of CO2 metabolism9,10,11. Here we show, in leaves of sunflower (Helianthus annuus L.), that stress decreases CO2 assimilation more than it slows O2 evolution, and that the effects are not reversed by high concentrations of CO212,13. Stress decreases the amounts of ATP9,11 and ribulose bisphosphate found in the leaves, correlating with reduced CO2 assimilation11, but the amount and activity of ribulose bisphosphate carboxylase-oxygenase (Rubisco) do not correlate. We show that ATP-synthase (coupling factor) decreases with stress and conclude that photosynthetic assimilation of CO2 by stressed leaves is not limited by CO2 diffusion but by inhibition of ribulose biphosphate synthesis, related to lower ATP content resulting from loss of ATP synthase.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lawlor,D. W. & Uprety,D. C. in Photosynthesis, Photoreactions to Plant Productivity (eds Abrol, Y. P., Mohanty, P. & Govindjee) 421–445 (Oxford and IBH, New Delhi, 1991).
Lawlor,D. W. in Environment and Plant Metabolism (ed. Smirnoff, N.) 129–160 (Bios Scientific, Oxford, 1995).
Cornic,G. & Massacci,A. in Photosynthesis and the Environment (ed. Baker, N. R.) 347–366 (Kluwer, The Netherlands, 1996).
Cornic,G. in Photoinhibition of Photosynthesis (eds Baker, N. R. & Bowyer, J. R.) 297–313 (Bios Scientific, Oxford, 1994).
Evans,L. T. Feeding the Ten Billion. Plants and Population Growth (Cambridge Univ. Press, Cambridge, 1998).
Ort,D. R., Oxborough,K. & Wise,R. R. in Photoinhibition of Photosynthesis (eds Baker, N. R. & Bowyer, J. R.) 315–329 (Bios Scientific, Oxford, 1994).
Brestic,M., Cornic,G., Fryer,M. J. & Baker,N. R. Does photorespiration protect the photosynthetic apparatus in French bean leaves from photoinhibition during drought stress? Planta 196, 450–457 (1995).
Quick,W. P. et al. The effect of water stress on photosynthetic carbon metabolisms in four species grown under field conditions. Plant Cell Environ. 15, 25–35 (1992).
Keck,R. W. & Boyer,J. S. Chloroplast response to low leaf water potentials. III. Differing inhibition of electron transport and photophosphorylation. Plant Physiol. 53, 474–479 (1974).
Younis,H. M., Boyer,J. S. & Govindjee Conformation and activity of chloroplast coupling factor exposed to low chemical potential of water in cells. Biochim. Biophys. Acta 548, 328–340 (1979).
Gimenez,C., Mitchell,V. J. & Lawlor,D. W. Regulation of photosynthetic rate of two sunflower hybrids under water stress. Plant Physiol. 98, 516–524 (1992).
Graan,T. & Boyer,J. S. Very high CO2 partially restores photosynthesis in sunflower at low water potentials. Planta 181, 378–384 (1990).
Lauer,M. J. & Boyer,J. S. Internal CO2 measured directly in leaves. Plant Physiol. 98, 1310–1316 (1992).
Pospíšilová,J. & Šantrúček,J. in Handbook of Photosynthesis (ed. Pessarakali, M.) 427–441 (Mercel Dekker, New York, 1997).
Gunasekera,D. & Berkowitz,G. A. Use of transgenic plants with ribulose 1,5-bisphosphate carboxylase/oxygenase antisense DNA to evaluate the rate limitation of photosynthesis under water stress. Plant Physiol. 103, 629–635 (1993).
Sage,R. F., Sharkey,T. D. & Seemann,J. R. The in vivo response of the ribulose-1,5-bisphosphate carboxylase activation state and the pool sizes of photosynthetic metabolites to elevated CO2 in Phaseolus vulgaris L. Planta 174, 407–416 (1988).
Price,G. D. et al. Chloroplast cytochrome b6/f and ATP synthase complexes in tobacco: transformation with antisense RNA against nuclear-encoded transcripts for the Rieske FeS and ATP δ polypeptides. Aust. J. Plant Physiol. 22, 285–297 (1995).
Sharkey,T. D. & Badger,M. R. Effects of water stress on photosynthetic electron transport, photophosphorylation and metabolite levels of Xanthium strumarium cells. Planta 156, 199–206 (1982).
Ortiz-Lopez,A., Ort,D. R. & Boyer,J. S. Photophosphorylation in attached leaves of Helianthus annuus at low water potentials. Plant Physiol. 96, 1018–1025 (1991).
Haraux,F. & de Kouchkovsky,Y. Energy coupling and ATP synthase. Photosynthesis Res. 57, 231–251 (1998).
Boyer,J. S., Chin Wong,S. & Farquhar,G. D. CO2 and water vapor exchange across leaf cuticle (epidermis) at various water potentials. Plant Physiol. 114, 185–191 (1997).
Jacob,J. & Lawlor,D. W. Dependence of photosynthesis of sunflower and maize leaves on phosphate supply, ribulose-1,5-bisphosphate carboxylase/oxygenase activity, and ribulose-1,5-bisphosphate pool size. Plant Physiol. 98, 801–807 (1992).
Paul,M. J. et al. Reduction in phosphoribulokinase activity by antisense RNA in transgenic tobacco: effect on CO2 assimilation and growth in low irradiance. Plant J. 7, 535–542 (1995).
Laisk,A. Calculation of leaf photosynthetic parameters considering the statistical distribution of stomatal apertures. J. Exp. Bot. 34, 1627–1635 (1983).
Terashima,I., Wong,S-C., Osmond,C. B. & Farquhar,G. D. Characterisation of non-uniform photosynthesis induced by abscisic acid in leaves having different mesophyll anatomies. Plant Cell Physiol. 29, 385–394 (1988).
Robinson,S. P., Grant,W. J. R. & Loveys,B. R. Stomatal limitations of photosynthesis in abscisic acid-treated and water-stressed leaves measured at elevated CO2. Aust. J. Plant Physiol. 15, 495–503 (1988).
Kozaki,A. & Takeba,G. Photorespiration protects C3 plants from photooxidation. Nature 384, 557–560 (1996).
Krause,G. H. & Weis,E. Chlorophyll fluorescence and photosynthesis: The basics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 313–349 (1991).
Sharp,R. E. & Boyer,J. S. Photosynthesis at low water potentials in sunflower: lack of photoinhibitory effects. Plant Physiol. 82, 90–95 (1986).
Edmonson,D. L., Kane,H. J. & Andrews,T. J. Substrate isomerization inhibits ribulosebisphosphate carboxylase-oxygenase during catalysis. FEBS Lett. 260, 62–66 (1990).
Riccardi,F., Gazeau,P., de Vienne,D. & Zivy,M. Protein changes in response to progressive water deficit in maize. Plant Physiol. 117, 1253–1263 (1998).
Schöffl,F., Prändl,R. & Reindl,A. Regulation of the heat-shock response. Plant Physiol. 117, 1135–1141 (1998).
Theobald,J. C., Mitchell,R. A. C., Parry,M. A. J. & Lawlor,D. W. Estimating the excess investment in ribulose-1,5-bisphosphate carboxylase/oxygenase in leaves of spring wheat grown under elevated CO2. Plant Physiol. 118, 945–955 (1998).
Acknowledgements
We acknowledge help in measurement of metabolites and Rubisco from A. J. Keys, M. A. Parry and M. J. Paul and of ATP-synthase by J. C. Theobald; discussions with R. A. C. Mitchell; and manuscript preparation by K. Lawlor. J. C. Gray supplied the ATP synthase antibody. Financial support to W.T. was from CONICYT, Venezuela and Rothamsted International. IACR-Rothamsted receives grant aid from the BBSRC, UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tezara, W., Mitchell, V., Driscoll, S. et al. Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 401, 914–917 (1999). https://doi.org/10.1038/44842
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/44842
This article is cited by
-
Plant endophytes: unveiling hidden applications toward agro-environment sustainability
Folia Microbiologica (2024)
-
Combined Application of Zinc and Silicon Improved Growth, Gas Exchange Traits, and Productivity of Maize (Zea mays L.) Under Water Stress
Silicon (2024)
-
ACC Deaminase Producing Phytomicrobiomes for Amelioration of Abiotic Stresses in Plants for Agricultural Sustainability
Journal of Plant Growth Regulation (2024)
-
Differential responses of contrasting low phosphorus tolerant cotton genotypes under low phosphorus and drought stress
BMC Plant Biology (2023)
-
Unveiling metabolic pathways involved in the extreme desiccation tolerance of an Atacama cyanobacterium
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.