Abstract
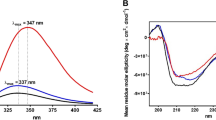
PROTEINS of the transferrin family, which contains serum transferri n and lactoferrin, control iron levels in higher animals through their very tight (Kapp ~1020) but reversible binding of iron1,2. These bilobate molecules3,4 have two binding sites, one per lobe, each housing one Fe3+ and the synergistic CO323– ion5. Crystallographic studies of human lactoferrin4,6 and rabbit serum transferrin7 in their iron-bound forms have characterized their binding sites and protein structure. Physical studies8,9 show that a substantial conformational change accompanies iron binding and release. We have addressed this phenomenon through crystal structure analysis of human apolactoferrin at 2.8 Å resolution. In this structure the N-lobe binding cleft is wide open, following a domain rotation of 53°, mediated by the pivoting of two helices and flexing of two interdomain polypeptide strands. Remarkably, the C-lobe cleft is closed, but unliganded. These observations have implications for transferrin function and for binding proteins in general.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Aisen, P. & Listowsky, I. A. Rev. Biochem. 49, 357–393 (1980)
Brock, J. H. in Metalloproteins Part 2 (ed. Harrison, P. M.) 183–262 (Macmillan, London, 1985).
Gorinsky, B. et al. Nature 281, 157–158 (1979).
Anderson, B. F. et al. Proc. natn. Acad. Sci. U.S.A. 84, 1769–1773 (1987).
Schlabach, M. R. & Bates, G. W. J. biol. Chem. 250, 2182–2188 (1975).
Anderson, B. F., Baker, H. M., Norris, G. E., Rice, D. W. & Baker, E. N. J. molec. Biol. 209, 711–734 (1989).
Bailey, S. et al. Biochemistry 27, 5804–5812 (1988).
Rossenau-Motreff, M. Y. F., Soetewey, R., Lamote, R. & Peeters, H. Biopolymers 10, 1039–1048 (1971).
Kilar, F. & Simon, I. Biophys. J. 48, 799–801 (1985).
Norris, G. E., Baker, H. M. & Baker, E. N. J. molec. Biol. 209, 329–331 (1989).
Bennett, W. S. & Huber, R. CRC Crit. Rev. Biochem. 15, 291–384 (1984).
Lesk, A. M. & Chothia, C. J. molec. Biol. 174, 175–191 (1984).
Baker, E. N., Rumball, S. V. & Anderson, B. F. Trends Biochem. Sci. 12, 350–353 (1987).
Kojima, N. & Bates, G. W. J. biol. Chem. 256, 12034–12039 (1981).
Cowart, R. E., Kojima, N. & Bates, G. W. J. biol. Chem. 257, 7560–7565 (1982).
Thornton, J. M. J. molec. Biol. 151, 261–287 (1981).
Kretchmar, S. A. & Raymond, K. N. J. Am. Chem. Soc. 108, 6212–6218 (1986).
Sack, J. S., Saper, M. A. & Quiocho, F. A. J. molec. Biol. 206, 171–191 (1989).
Kretchmar, S. A. & Raymond, K. N. Inorg. Chem. 27, 1436–1441 (1988).
Brown, J. P. et al. Nature 296, 171–173 (1982).
Mao, B., Pear, M. R., McCammon, J. A. & Quiocho, F. A. J. biol. Chem. 257, 1131–1133 (1982).
Crowther, R. A. in The Molecular Replacement Method (ed. Rossmann, M. G.) 173–178 (Gordon and Breach, New York, 1972).
Sussman, J. L. Meth. Enzymol. 115, 271–303 (1985).
Tronrud, D. E., Ten Eyck, L. F. & Matthews, B. W. Acta crystallogr. A43, 489–501 (1987).
Jones, T. A. J. appl. Crystallogr. 11, 268–272 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Andersen, B., Baker, H., Morris, G. et al. Apolactoferrin structure demonstrates ligand-induced conformational change in transferrins. Nature 344, 784–787 (1990). https://doi.org/10.1038/344784a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/344784a0
This article is cited by
-
Complex of human Melanotransferrin and SC57.32 Fab fragment reveals novel interdomain arrangement with ferric N-lobe and open C-lobe
Scientific Reports (2021)
-
Effect of apo-lactoferrin on leukotoxin and outer membrane vesicles of Mannheimia haemolytica A2
Veterinary Research (2020)
-
Accurate flexible refinement of atomic models against medium-resolution cryo-EM maps using damped dynamics
BMC Structural Biology (2018)
-
Structure and domain dynamics of human lactoferrin in solution and the influence of Fe(III)-ion ligand binding
BMC Biophysics (2016)
-
Exploring the Fe(III) binding sites of human serum transferrin with EPR at 275 GHz
JBIC Journal of Biological Inorganic Chemistry (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.