Abstract
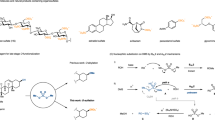
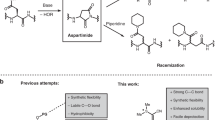
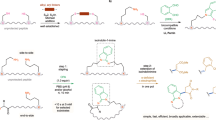
A STUDY of the mechanism of the phenyl isothiocyanate degradation of peptides1 has made necessary a revision of our earlier formulation of this reaction. It has been found that the compound initially formed in the acid-catalysed cleavage of a phenyl thiocarbamyl peptide is not the expected 3-phenyl-2-thiohydantoin derivative of the N-terminal amino-acid, but instead the isomeric 2-anilino-5-thiazolinone.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Edman, P., Arch. Biochem., 22, 475 (1949); Acta Chem. Scand., 4, 283 (1950).
Ottesen, M., and Wollenberger, A., Nature, 170, 801 (1952). Fraenkel-Conrat, H., and Fraenkel-Conrat, J., Acta Chem. Scand., 4, 1409 (1950).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
EDMAN, P. Mechanism of the Phenyl Isothiocyanate Degradation of Peptides. Nature 177, 667–668 (1956). https://doi.org/10.1038/177667b0
Issue Date:
DOI: https://doi.org/10.1038/177667b0
This article is cited by
-
Determination of sequence and absolute configuration of peptide amino acids by HPLC–MS/CD-based detection of liberated N-terminus phenylthiohydantoin amino acids
Scientific Reports (2022)
-
The influence of commonly used tags on structural propensities and internal dynamics of peptides
Monatshefte für Chemie - Chemical Monthly (2019)
-
Close genetic relationship between Nitrobacter hamburgensis nitrite oxidoreductase and Escherichia coli nitrate reductases
Archives of Microbiology (1993)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.