Abstract
As personal care products (PCPs) are used in close contact with a person, they are a major source of consumer exposure to chemical substances contained in these products. The estimation of realistic consumer exposure to substances in PCPs is currently hampered by the lack of appropriate data and methods. To estimate aggregate exposure of consumers to substances contained in PCPs, a person-oriented consumer exposure model has been developed (the Probabilistic Aggregate Consumer Exposure Model, PACEM). The model simulates daily exposure in a population based on product use data collected from a survey among the Dutch population. The model is validated by comparing diethyl phthalate (DEP) dose estimates to dose estimates based on biomonitoring data. It was found that the model’s estimates compared well with the estimates based on biomonitoring data. This suggests that the person-oriented PACEM model is a practical tool for assessing realistic aggregate exposures to substances in PCPs. In the future, PACEM will be extended with use pattern data on other product groups. This will allow for assessing aggregate exposure to substances in consumer products across different product groups.
Similar content being viewed by others
INTRODUCTION
Exposure to Chemical Substances in PCPs
The use of personal care products (PCPs) is widespread in the population. As these products are used in close contact with a person, PCPs are a source of potentially high exposure to chemical substances contained in these products.
The assessment of realistic exposures to chemicals in PCPs is complicated by the heterogeneity in the use and chemical composition of these products. Of special consideration is the situation when a substance is included in a whole array of products and the total or aggregate exposure from these products has to be determined, rather than just the exposure arising from a single product.
For risk-assessment purposes, the assessor often has to rely on crude assumptions regarding the use of PCPs and their composition. When determining aggregate exposure, however, using the simplistic method of adding up the potential exposures from all products containing a substance may quickly lead to gross overestimation of the exposure levels in the population.1 Although this method can be acceptable for low-tier, screening risk assessments, such methods are unsuited for situations in which more realistic estimations of the exposure are required.
In an assessment of the aggregate exposure to parabens in PCPs, Cowan-Ellsberry and Robison2 tried to partly solve the problem by introducing expert-judgement-based modifying factors. These factors were assumed to account for co-use and non-use patterns of products and for the variation in paraben content of the products. The approach, however, suffers from a high degree of arbitrariness and it remains unclear how suitable modifying factors should be chosen.
Using an alternative approach, Wormuth et al.3 assessed exposure to diethyl phthalate (DEP), including PCPs as the major source using a scenario-based approach. In this method, ranges (characterized as high, medium and low) of population exposure were estimated. Estimation of these ranges was based on ranges in various exposure determinants, but the method of aggregation still consisted in the simple adding up of potential exposures from products, neglecting the details on product use and co-use.
A more systematic approach to the estimation of aggregate exposures from different products is the method of person-oriented modeling.4, 5, 6 This method requires detailed data on the use and co-use of PCPs, and such information is not widely available.
McNamara et al.7 and Hall et al.8 collected PCP use data combining market information databases with a controlled product use study. The market information study contained a large number of users (male and female) of the studied products in five European countries. The study included a number of PCPs: body lotion, shampoo, deodorant spray, deodorant non-spray, facial moisturiser, lipstick and toothpaste. The information collected is not publicly available in a format that is usable in aggregate exposure assessments.
A postal survey on PCP use among the German-speaking Swiss population was recently conducted.9 The survey collected information for eight leave-on products on the number of individuals in the population that use the product. The survey included both children and adults. Product use in Switzerland was compared with German and Dutch product use data.10 Slight but significant differences were observed between the Swiss population on the one hand and German and Dutch populations on the other. However, product use by German and Dutch populations was similar.
In the absence of appropriate, publicly available PCP use data at the start of this work, a survey on PCP use in the Netherlands was conducted.10 Data were collected on the use frequency and the use amounts of 32 PCPs among a survey population of 512 Dutch adults (210 male and 302 female). The data were used to develop a person-oriented exposure model — the Probabilistic Aggregate Consumer Exposure Model (PACEM).
In an earlier publication, it was described how PACEM was applied to estimate the exposure to parabens in PCPs as an example of how person-oriented modeling compares with low-tier exposure assessment.1
In this paper, the PACEM exposure estimates are compared with an exposure evaluation based on biomonitoring as an independent verification of the modeling approach.
For this verification, DEP was chosen. DEP is used as a solvent and a vehicle for fragrance and cosmetic ingredients.11 Several studies have shown that PCPs are the main source of population exposure to DEP.3, 12, 13, 14, 15, 16 Therefore, to a large extent, biomonitoring data reflects exposures arising from the use of PCPs.
In addition, there exists ample biomonitoring data on representative populations for DEP.17, 18, 19 A method to relate biomarker data to DEP exposure is also provided.17 These conditions make DEP a suitable substance to directly verify the person-oriented exposure estimation method.
METHODS
PACEM: Outline of the Modeling Approach
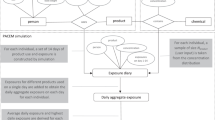
In person-oriented exposure modeling the exposed person is taken as the central entity in the exposure calculation.4, 5, 6 Aggregation is performed by adding-up of the product exposures based on the exposed person’s product use pattern. In principle, person-oriented modeling can be deterministic, in which case the person represents a homogeneous group of product users sharing a common pattern of product use. More often, however, person-oriented models are probabilistic and the exposed person is taken to represent a specific individual in an exposed population. By taking the exposed person rather than the product as the central unit of exposure evaluation, unrealistic combinations of product exposures that will not co-occur in reality are prevented.
PACEM was developed to facilitate realistic exposure assessment of substances in PCPs. PACEM is a probabilistic exposure model. The central data in the model are the data obtained from a population survey on product use.10 The data were collected in a web-based questionnaire among 512 Dutch adults (210 male and 302 female). Use frequencies and used amounts were recorded for 32 PCPs. In addition, person information such as body weight, age and gender were collected. The data on used amounts and product use frequencies are summarized in Table 1.
The PACEM exposure model estimates exposure by repeatedly sampling individual product use and person data from the survey database and combining these with information on the concentration of the substance in products. This method estimates total daily exposures for a sample of individuals based on realistic product use data. These exposure estimates are expected to reflect to a high extent the realistic exposures of persons in the population to multiple PCPs.
Person-Oriented Population Model: Details
In detail, the model takes the following steps:
-
1)
From the product use data obtained from the survey, a model population of N individuals is constructed. This is done by repeatedly sampling the data on the survey population. Each individual in the model population is a copy of a person in the survey population, having the same use frequency and used amount for each product the person uses, and also the same personal characteristics (e.g., body weight, age and gender).
-
2)
For each individual i in the model population, the dose Dij from exposure to a substance in PCPs on a single day j is modeled as:

In this equation, summation is over all products that the person uses.
Aik is the amount of product k that the individual i uses. wfk is the concentration of the substance in the product k. wfk is sampled from concentration data on substance in the products. The product concentration data may either be a collection of raw data on monitored products or it may be sampled from parametric concentration distributions derived from monitoring data or other data sources.
nijk is the number of uses of the product k by person i on day j. This is determined by random sampling from the survey data as follows: if the use frequency for person i of the product k is less than once a day, a random number RN between 0 and 1 is sampled from a uniform probability distribution. If RN is smaller than the use frequency (per day), nijk is set to 1 (i.e., the product k is used on this particular day j), and to zero otherwise (no use on this particular day). Product use frequencies in the survey that are larger than once a day are specified as integers. If the use frequency of the product k for person i is larger than once a day, nijk is set equal to the use frequency.
efk is the ‘exposure fraction’, the fraction of the substance from product k that is actually absorbed by the individual. Exposure fractions are estimated for each substance separately and provided as input to PACEM.
Finally, the sum over the absorbed amounts from all different product exposures is divided by the individual’s body weight BWi to obtain the dose on day j for this individual.
-
1)
Step 2 is repeated (if needed) for the number of days M in the exposure period that is considered.
-
2)
Steps 2 and 3 result in a set of M daily doses for N individuals in the population. From this set, different measures of population exposure can be derived, such as the distribution of long-term averaged daily exposure or any specific percentile of this.
Person-Specific Data on PCP Use Patterns
Essential data on the use of PCPs was collected from a survey, the results of which have been published elsewhere.10 The products that were included in the survey are listed in Table 1.
Model Implementation
The stochastic sampling, the calculation of the event exposures and the combination of data in PACEM is implemented in the R modeling language. The relational database that includes the PCP use data, the data on the surveyed individuals and the product composition data was built as an MS Access database.
Modeling the Exposure of the Dutch Population to DEP
The model described above was used to estimate the exposure of the Dutch population to DEP in PCPs. To apply the model, the following specific information for DEP is needed:
-
1)
An estimate of the amount of DEP in the PCPs used in the population.
-
2)
An estimate of the exposure fraction for each product.
Concentration Data of DEP in PCPs
Data on the concentration of DEP in PCPs were obtained from public literature: Koniecki et al.20 (Canada); Houlihan et al.21 (USA); DiGangi et al.22 (Sweden); TNO report23 (UK/The Netherlands); and Dodson et al.24 (USA). This data was used to estimate two characteristics of the concentration of DEP in PCPs: first, the fraction of products that do not contain DEP (fraction of zeros) and, second, for the products that contain DEP, the parametric distribution function of the product concentrations in the PCPs.
For fragrance, hair care, deodorant, nail polish, (body) lotion and skin cleanser, detailed surveys were performed to obtain DEP concentrations. For these products, there was sufficient information to determine the fraction of products not containing DEP and to specify a concentration distribution for the products containing DEP (Table 2). This distribution was assumed to be lognormal and completely defined by the geometric mean (GM) and geometric SD (GSD). The GM and GSD were determined as the back-transformed mean and the SD of the log-transformed raw concentration data. For the remaining products, no detailed concentration data were available. The study by Dodson et al.24 was used to estimate upper and lower limits of the DEP product concentrations. The concentrations were described by a uniform probability distribution between these limits. The products were assigned a fraction of zeroes of 0.2. This fraction was chosen from the range of fractions (i.e., between 0.1 and 0.7) from Table 2. Afterwards, a sensitivity analysis was conducted for this assumption to assess its impact on the exposure estimates. Calculations of the population exposure were repeated assuming a fraction of zeroes of 0.1 and 0.7, respectively. Changes in the calculated percentiles of the dose distribution were within 10% for this range of parameter values (data not shown). As a result, the impact of this assumption on the dose estimates was considered to be low. The assumptions for the DEP concentration in these products are summarized in Table 3.
Using the distribution functions defined with these parameters to represent the concentration data, for each of the 32 PCPs 100 DEP concentrations were sampled, representing a set of realistic products.
Exposure Fractions for DEP from PCPs
Exposure to DEP in PCPs is expected to occur mainly from dermal application of products, as most PCPs are applied on the skin and the evaporation potential of DEP is low (the vapor pressure of DEP is 0.00165 mm Hg at 25 °C).
Dermal absorption will depend on the surface load of DEP on the skin, the duration of the exposure and the permeability of the skin to DEP.
Using the permeability coefficient P, the amount that is dermally absorbed is estimated as:

Aabs is the amount of substance absorbed through the skin. Aexp is the amount of substance on the skin. S is the exposed surface area of the skin. C the concentration of the substance in the matrix on the skin. V is the volume of the product on the skin. Finally, t is the exposure duration.
To estimate the permeability coefficient P, a model proposed by Mitragotri25 was used. This model was found to give the best results in an evaluation of several dermal permeability models.26 The model predicts a permeability of the stratum corneum of 0.0028 cm/h, which was taken as the permeability of the skin. The product-specific assumptions for S and t were taken from the defaults database of the ConsExpo27 consumer exposure assessment tool. The justification and background of these assumptions is provided in the RIVM Cosmetics fact sheet.28 For the concentration C of the substance on the skin, the assumption was made that the substance was in its pure form (i.e., a DEP mass density of 1.1 g/cm3 was used).
The exposure fractions that were thus estimated are given in Table 4.
Simulating the Daily Dose of DEP in the Population
PACEM was used to simulate the dose for DEP in the population on a single day. This dose estimate is expected to be comparable to estimates of the daily dose derived from biomonitoring data available for DEP. To estimate the dose distribution for DEP, a population of 10,000 individuals was sampled from the product use data included in PACEM. Based on the use frequency data, the products each individual used on the selected day were determined. For every use of the product, the DEP dose was estimated and all product exposures for one individual were added up. This resulted in a distribution of aggregate DEP doses over the population on the modeled day.
RESULTS
A histogram of the total DEP dose in the population on a single day is shown in Figure 1. Figure 2 presents the cumulative dose distribution for men, women and the total population separately. The percentages of the population that were not exposed on a particular day are 5.7% (men=4.6% and women=1.1%). Details of the exposure distribution are provided in Table 5.
Cumulative distributions of single-day diethyl phthalate (DEP) exposures. Solid line: men and women. Dashed line (left): men; dotted line (right): women. Vertical lines represent DEP dose estimates from the biomonitoring data by Koch et al.17 Solid line: median; dashed line: 95th percentile.
The estimated dose shows a large variability among individuals in the population, varying roughly three orders of magnitude for the exposed individuals.
For the population as a whole on the modeled day, the estimated median DEP dose was 4.1 μg/kg. For the subpopulation of men it was 2.3 μg/kg, and for women a value of 5.4 μg/kg was estimated. The estimated 95th percentiles were 67.8 μg/kg for the total population, 63.3 μg/kg for men and 71.2 μg/kg for women.
These DEP dose estimates can be used to verify the model predictions with the dose estimates based on biomonitoring data.
From a detailed estimate of the DEP dose distribution in the population derived using PACEM, a lot more information can be obtained. For example, Figure 3 provides information on the contribution of the various PCPs to the total dose. This demonstrates that the largest contributors to exposure for the entire population are body lotion, aftershave balsam, perfume and deodorant spray.
Estimation of the DEP Dose from Biomonitoring Data
For DEP, several biomonitoring studies have been conducted. Of these, the studies by Koch et al.,17 Koch and Calafat,18 and Ye et al.19 were taken as reference. These studies monitored DEP exposures in German and Dutch populations. Exposure in these populations is expected to be roughly similar and data from these studies are deemed suitable for a verification of the modeled DEP exposures in the Dutch population. Data on the Dutch population was on (pregnant) women only.19 As this data compares well with the data on the German female population (see Table 6), it was assumed that indeed data on the DEP dose in the general. German population could be taken to represent the DEP dose in the Dutch population as well. All studies monitored the metabolite MEP of DEP in urine. Daily doses of DEP were inferred from MEP levels in urine spot samples, with a method described in Koch et al.17:

In this equation, UE is the urinary excretion of MEP per amount of creatinine, CE the creatinine excretion rate normalized by body weight, set to 18 mg/kg/day for women and 23 mg/kg/day for men, FUE is the molar fraction of the urinary excreted monoester related to the ingested diester. For MEP, FUE was estimated to be 0.64 (hypothesized to be the same as the fractional urinary excretion of MnBP after DnBP intake, which was determined experimentally). Finally, MWd and MWm are the molecular weight of the diester and monoester, respectively. The method assumes essentially steady-state conditions of exposure and elimination. Strictly speaking, the assumption of steady-state conditions is only justified if the temporal variation in PCP use over the day is short compared with the elimination half-life time of DEP from the body. As the latter is relatively short (2–3 h), the assumption of steady state may not be completely accurate.
Koch et al.17 derived daily doses of DEP in a population of 85 individuals including children (aged 7 years and older) as well as adults (aged up to 63 years). In this group, the median (95th percentile) DEP dose was 2.3 (22.1) μg/kg. For the adult males (n=25) and females (n=34), the daily DEP doses were determined as 2.4 (20) and 4.4 (33.6) μg/kg, respectively. Koch and Calafat14 only monitored adults. In this group, the median (95th percentile) DEP dose was 1.9 (9.7) μg/kg. Ye et al.13 monitored pregnant women in the Netherlands. Median (95th percentile) dose in this population was 2.9 (28.1) μg/kg.
The DEP exposure estimates obtained from these studies have been summarized in Table 6.
In a comparison with these data, the hypothesis that PCPs present the largest source of DEP exposure can be verified. For example, Clark et al.29 estimate that the median combined dose to DEP exposure via the ingestion of food, drinking water, dust and inhalation of air is 0.46 μg/kg/day, with a 95th percentile of 1.0 μg/kg/day. As a result, it can be seen that this exposure represents only a small part of the total exposure.
DISCUSSION
Comparing the PACEM Dose Estimate with Dose Estimates Based on Biomonitoring Data
To verify the exposure modeling approach, the dose simulated for a single day was compared with the daily dose of DEP derived from biomonitoring data. For the general population, the modeled median is 4.1 μg/kg versus median values of 2.3 and 1.9 μg/kg estimated from the biomonitoring studies. The model’s estimates of the central tendencies of the population exposure are slightly higher than the estimates based on biomonitoring, but in good agreement. By contrast, the modeled 95th percentile of 67.8 μg/kg is markedly higher (a factor of 3 to 10) than the values obtained from biomonitoring (22.1 and 9.7 μg/kg).
A similar pattern is observed when considering the male and female subpopulations separately. The median dose estimates made with PACEM (2.3 μg/kg for men and 5.4 μg/kg for women) are comparable to (but somewhat higher than) the median values estimated in Koch et al.17 (2.4 μg/kg for men and 4.4 μg/kg for women). The modeled 95th percentiles of the single day dose of 63.3 μg/kg (men) and 71.2 μg/kg (women) are higher than the 95th percentiles of the dose found by Koch et al.17 (20 μg/kg for male and 33.6 μg/kg for female populations).
For the comparison of exposure modeling with the estimates derived from biomonitoring data, it should be noted that both methods of exposure estimation are subject to uncertainty.
Uncertainty in the Modeling of DEP Exposure
The uncertainty in the modeling stems predominantly from the estimate of the dermal absorption of DEP. To estimate skin permeability, the Mitragotri25 model was used. This model has been evaluated in Lian et al.26 For a set of 124 chemical compounds the predicted skin permeability was compared with experimental permeability data. For substances that are not too hydrophilic (Kow>0.01), the predicted permeability values were within two orders of magnitude of the experimental values. This uncertainty in the permeability translates into uncertainty in the evaluated product exposure of up to a (maximum) factor of 100 (depending on the product). As the magnitude of the uncertainty will vary from product to product, the total uncertainty in the exposure due to uncertainty in the skin permeability is in all likelihood smaller than this maximum factor of 100. Still, this is a significant source of uncertainty.
In addition, it was assumed that the absorption through the skin was essentially from pure DEP. In reality, however, the applied product will be a more complex matrix, consisting of different product components. DEP absorption from this matrix may not be adequately represented by absorption from pure DEP. In the first place, the partitioning of DEP between the skin and the product matrix may be different from that between skin and pure DEP. Moreover, diffusion of DEP in the product matrix to the skin may become an important factor. In this situation, dermal absorption may become dependent on the thickness of the layer of product on the skin. As both general product compositions and the effect of the matrix on the dermal absorption are largely unknown, these aspects were not considered and add to the overall uncertainty in the evaluation.
Other sources of uncertainty in the modeling include uncertainty in the specified amounts and frequencies in the survey on product use, and uncertainty in the (distribution of the) product composition (i.e., the weight fractions of DEP in the products). These uncertainties, however, are deemed to be much smaller than the one that stems from the skin permeability.
Uncertainty in the DEP Exposure Estimate Based on Biomonitoring Data
The estimates based on biomonitoring data, on the other hand, have their own uncertainties.
Aylward et al.30 analyzed the uncertainty in the method that is used to relate MEP urine concentrations to oral DEP intake, recommended by Koch et al.17 The method assumes steady-state exposure and excretion conditions. Owing to temporal variations in DEP exposure and the relatively short elimination half-life time of DEP from the body of 2–3 h, the variation in DEP doses inferred from urine spot samples may be underestimated. Aylward et al.30 performed a model study on the effect of elimination kinetics on the estimated variation in DEP exposure from spot urine samples using a first-order kinetics model. The effect of elimination kinetics on the inferred range of DEP doses in the population was found to be relatively small: the ratio of the 95th and 50th percentiles of the DEP dose was expected to be underestimated by at most 50%.
In addition to this uncertainty in the method of relating MEP biomarker data to actual DEP dose, there is statistical uncertainty in the biomarker data due to the limited sample size. The values reported in Koch et al.17 refer to people in the age ranges of 7–63 years. The total size of the population was 85. The sizes of the adult male and adult female subpopulations were 25 and 34, respectively. Owing to the limited sample size, the uncertainty in particularly the estimated 95th percentile will be high.
Finally, the biomonitoring data comprises all sources of exposure, whereas PACEM considered only the DEP exposure from PCPs. It has already been discussed that exposure via food and air is negligible compared with the exposure from PCPs.31 Another source of DEP exposure, not considered so far, is exposure via the intake of medicines.32 This source of DEP exposure is potentially high, but hard to quantify.
Comparison of Modeling with Biomonitoring Data
Given the uncertainty in both the exposure modeling and the biomonitoring data, the two methods agree well and are judged to be in accordance.
CONCLUSIONS
In this work, the results of a person-oriented exposure model PACEM were verified by comparing estimated daily doses of DEP in PCPs with dose estimates based on biomonitoring data. Given the uncertainties in both the biomonitoring and the modeling of DEP exposure, the results of the two methods are in good agreement. This supports the view that the person-oriented method employed in this work yields reliable results and should be the preferred method in realistic exposure assessment practice.
In order to assess realistic exposures of consumers to substances in consumer products, the model should be extended with use pattern data for other product groups such as household products, do-it-yourself products and pest control products. This would then allow assessment of realistic exposures not only from substances used in these product groups, but also from substances contained in products belonging to several product groups.
PACEM is currently based exclusively on Dutch PCP use data. The incorporation of data from surveys of other (European) countries will greatly enhance the significance of the model.
References
Gosens I, Delmaar JE, ter Burg W, de Heer C, Schuur AG . Aggregate exposure approaches for parabens in personal care products: a case assessment for children between 0-3 years old. J Expo Sci Environ Epidemiol 2014: 24: 208–214.
Cowan-Ellsberry CE, Robison SH . Refining aggregate exposure: example using parabens. Regul Toxicol Pharmacol 2009: 55: 321–329.
Wormuth M, Scheringer M, Vollenweider M, Hungerbuhler K . What are the sources of exposure to eight frequently used phthalic acid esters in europeans? Risk Anal 2006: 26: 803–824.
Arnold SF, Price PS . The LifeLine Group, modeling mixtures resulting from concurrent exposures to multiple sources. Toxicol Appl Pharmacol 2007: 223: 121–124.
Price PS, Chaisson CF . A conceptual framework for modeling aggregate and cumulative exposures to chemicals, 2005. J Expo Anal Environ Epidemiol 2005: 15: 473–481.
Zartarian VG, Özkaynak H, Burke JM, Zufall MJ, Rigas ML, Furtaw EJ Jr . A modeling framework for estimating children’s residential exposure and dose to chlorpyrifos via dermal residue contact and nondietary ingestion. Environ Health Perspect 2000: 108: 505–514.
McNamara C, Rohan D, Golden D, Gibney M, Hall B, Tozer S et alProbabilistic modeling of European consumer exposure to cosmetic products. Food Chem Toxicol 2007: 45: 2086–2209.
Hall B, Tozer S, Safford B, Coroama M, Steiling W, Leneveu-Duchemin MC et alEuropean consumer exposure to cosmetic products, a framework for conducting population exposure assessments. Food Chem Toxicol 2007: 45: 2097–2108.
Manová E, von Goetz N, Keller C. Use patterns of leave-on personal care products among Swiss-German children, adolescents, and adults. Int J Env Res Public Health 2014: 10: 2778–2798.
Biesterbos JWH, Dudzina T, Delmaar JE, Bakker MI, Russel FGM, von Götz N et alUsage patterns of personal care products: important factors for exposure assessment. Food Chem Toxicol 2013: 55: 8–17.
Api AM . Toxicological profile of diethyl phthalate: a vehicle for fragrance and cosmetic ingredients. Food Chem Toxicol 2001: 39: 97–108.
Just AC, Adibi JJ, Rundle AG, Calafat AM, Camann DE, Hauser R et alUrinary and air phthalate concentrations and self-reported use of personal care products among minority pregnant women in New York city. J Expo Sci Environ Epidemiol 2010: 20: 625–633.
Parlett LE, Calafat AM, Swan SH . Women’s exposure to phthalates in relation to use of personal care products. J Expo Sci Environ Epidemiol 2013: 23: 197–206.
Romero-Franco M, Hernández-Ramírez RU, Calafat AM, Cebrián ME, Needham LL, Teitelbaum S et alPersonal care product use and urinary levels of phthalate metabolites in Mexican women. Environ Int 2011: 37: 867–871.
Buckley JP, Palmieri RT, Matuszewski JM, Herring AH, Baird DD, Hartmann KE et alConsumer product exposures associated with urinary phthalate levels in pregnant women. J Expo Sci Environ Epidemiol 2013: 22: 468–475.
Koch HM, Lorber M, Christensen KLY, Pälmke C, Koslitz S, Brüning T . Identifying sources of phthalate exposure with human biomonitoring: Results of a 48 h fasting study with urine collection and personal activity patterns. Int J Hyg Environ Health 2013: 216: 672–681.
Koch HM, Drexler H, Angerer J . An estimation of the daily intake of di 2-ethylhexyl phthalate (DEHP) and other phthalates in the general population. Int J Hyg Environ Health 2003: 206: 77–83.
Koch HM, Calafat AM . Human body burdens of chemicals used in plastic manufacture. Phil Trans R Soc B 2009: 364: 2063–2078.
Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM et alUrinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, The Netherlands: The Generation R study. Environ Res 2008: 108: 260–267.
Koniecki D, Wang R, Moody RP, Zhu J . Phthalates in cosmetic and personal care products: concentrations and possible dermal exposure. Environ Res 2011: 111: 329–336.
Houlihan J, Brody C, Schwan B . Not Too Pretty Phthalates, Beauty Products & the FDA. Environmental Working Group USA, 2002.
DiGangi J, Norin H Pretty Nasty—Phthalates in European Cosmetic Products. Health Care Without Harm, 2002.
TNO-report. Hazardous Chemicals in Consumer Products. Nr. 52003227 2003.
Dodson RE, Nishioka M, Standly LJ, Perovich LJ, Brody JG, Rudel RA . Endocrine disruptors and asthma-associated chemicals in consumer products. Environ Health Perspect 2012: 120: 935–943.
Mitragotri S . A theoretical analysis of permeation of small hydrophobic solutes across the stratum corneum based on Scaled Particle Theory. J Pharm Sci 2002: 91: 744–752.
Lian G, Chen L, Han L . An evaluation of mathematical models for predicting skin permeability. J Pharm Sci 2008: 97: 584–598.
Delmaar JE, van der Zee Park M, van Engelen JGM . ConsExpo—Consumer Exposure and Uptake Models -Program Manual RIVM report 320104004 2005. www.rivm.nl.
Bremmer HJ, Prud'Homme de Lodder LCH, van Engelen JG, Cosmetics Fact Sheet. To assess the risks for the consumer. Updated version for ConsExpo 4. RIVM Report 320104001 2006. www.rivm.nl.
Clark KE, David RM, Guinn R, Kramarz KW, Lamp MA, Staples CA . Modeling human exposure to phthalate esters: a comparison of indirect and biomonitoring estimation methods. Hum Ecol Risk Assess 2011: 17: 923–965.
Aylward LL, Kirman CR, Adgate JL, McKenzie LM, Hays SM . Interpreting variablity in population biomonitoring data: Role of elimination kintecis. J Expo Sci Environ Epidemiol 2012: 22: 398–408.
Fromme H, Schlummer M, Möller A, Gruber L, Wolz G, Ungewiss J et alExposure of an adult population to perfluorinated substances using duplicate diet portions and biomonitoring data. Environ Sci Technol 2007: 4: 7928–7933.
Kelley KE, Hernandez-Diaz S, Chapllin EL, Hauser R, Mitchell AA . Identification of phthalates in medications and dietary supplement formulations in the United States and Canada. Environ Health Perspect 2012: 120: 379–384.
Acknowledgements
We gratefully acknowledge the financial support of the Dutch Ministry of Health, Welfare and Sports within the scope of the ‘Kennisvraag 5.1.3’ programme. We also thank Tatsiana Dudzina and Nathalie von Goetz of the Swiss Federal Institute of Technology (ETHZ) in Zurich, and Jacqueline Biesterbos and Nel Roeleveld of the Radboud University Nijmegen Medical Centre (RUNMC) for their indispensable contributions to the development of the PACEM exposure model that was used in this work, and Sang Hee Lee of the Korean National Institute of Environmental Research (NIER) for her contribution in the early stages of this study. This work was financially supported by order and for the account of the Dutch Ministry of Health, Welfare and Sports.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Delmaar, C., Bokkers, B., ter Burg, W. et al. Validation of an aggregate exposure model for substances in consumer products: a case study of diethyl phthalate in personal care products. J Expo Sci Environ Epidemiol 25, 317–323 (2015). https://doi.org/10.1038/jes.2014.68
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jes.2014.68
Keywords
This article is cited by
-
PACEMweb: a tool for aggregate consumer exposure assessment
Journal of Exposure Science & Environmental Epidemiology (2023)
-
Exposure to selected preservatives in personal care products: case study comparison of exposure models and observational biomonitoring data
Journal of Exposure Science & Environmental Epidemiology (2020)
-
Consumer product chemical weight fractions from ingredient lists
Journal of Exposure Science & Environmental Epidemiology (2018)
-
Stochastic modeling of near-field exposure to parabens in personal care products
Journal of Exposure Science & Environmental Epidemiology (2017)