Abstract
Pseudomonas aeruginosa is an opportunistic pathogen that can be very hard to treat because of high resistance to different antibiotics and alternative treatment regimens are greatly needed. An alternative or a complement to traditional antibiotic is to inhibit virulence of the bacteria. The salicylidene acylhydrazide, INP0341, belongs to a class of compounds that has previously been shown to inhibit virulence in a number of Gram-negative bacteria. In this study, the virulence blocking effect of INP0341 on P. aeruginosa was studied in vitro and in vivo. Two important and closely related virulence system were examined, the type III secretion system (T3SS) that translocates virulence effectors into the cytosol of the host cell to evade immune defense and facilitate colonization and the flagella system, needed for motility and biofilm formation. INP0341 was shown to inhibit expression and secretion of the T3SS toxin exoenzyme S (ExoS) and to prevent bacterial motility on agar plates and biofilm formation. In addition, INP0341 showed an increased survival of P. aeruginosa-infected mice. In conclusion, INP0341 attenuates P. aeruginosa virulence.
Similar content being viewed by others
Introduction
Pseudomonas aeruginosa is a Gram-negative pathogen that utilizes a large set of virulence factors1 to cause infections in human hosts that vary from asymptomatic to acute systemic infections. As it causes disease primarily in individuals whose health is compromised in some manner, it is considered an opportunistic pathogen.2, 3 In most cases of infection, the integrity of a physical barrier to infection is lost; for example, skin and mucous membranes or an underlying immune deficiency. These include patients with severe burns, cancer, AIDS, cystic fibrosis, wounds or chronic conditions that require prolonged hospitalization.4, 5 Once a P. aeruginosa infection is established, it often poses a therapeutic dilemma because of intrinsic and acquired antibiotic resistance.6 Compared with other Gram-negative bacteria, P. aeruginosa is naturally less susceptible to most antibiotics, such as β-lactams, chloramphenicol and quinolones. P. aeruginosa harbors an intrinsic resistance because of low penetration across the outer membrane, multidrug efflux pumps that actively transport antibiotics out of the bacteria and chromosomally encoded a β-lactamase.7, 8 In addition, the bacteria have a highly charged membrane and can form biofilms that are organized communities of bacteria that make them much more resistant to antibiotics.9 By means of mutational changes or acquisition of exogenous genetic material, P. aeruginosa has the ability to develop resistance to all known antibiotics that are active against P. aeruginosa. Furthermore, these acquired resistance mechanisms make it possible for P. aeruginosa to develop resistance to antibiotics during ongoing therapy.10
Biofilms are highly structured complex communities with a distinct architecture consisting of bacteria embedded in a self-secreted extracellular polysaccharide matrix or slime attached to a surface.11, 12 The biofilm has a protective effect for the bacteria as a result of an adaptive response. In addition to reduced susceptibility to antibiotics, the milieu in a biofilm also protects the bacteria against environmental stresses and phagocytosis by the host immune response.13, 14 Biofilm formation is dependent on production of functional surface organelles necessary for virulence, motility and adherence, namely flagella and type IV pili.15 P. aeruginosa uses the two surface organelles, either separately or together for motility depending on the surrounding. Movement can, for example, appear as swimming, a movement that only requires functional flagellum, or swarming that requires both functional flagellum and type IV pilus.16, 17
Another important virulence system in P. aeruginosa is the type III secretion system (T3SS) that is required for colonization and survival in the host.1, 18, 19 Many Gram-negative disease-causing bacteria harbor the T3SS for delivery of effector molecules into the host cells, including Shigella spp., Salmonella spp., Yersinia spp., Chlamydia spp., enteropathogenic and enterohaemorrhaghic Escherichia coli and P. aeruginosa.20, 21, 22, 23 The T3SS in P. aeruginosa is induced when the bacterium enters a host and following close contact with eukaryotic cells the system mediate translocation of four effector molecules, exoenzyme (Exo) S, T, U and Y, from the bacterial cytosol directly into the host cell cytoplasm where they alter different functions of the cells in order to make it possible for the bacteria to grow.24, 25, 26, 27 The T3SS is active during early infection and when the infection proceeds the T3SS is downregulated and biofilm formation along with production of extracellular matrix starts.28
Previous studies have shown that the T3SS and the flagella system have key functions when P. aeruginosa infects burn wounds or lungs.29, 30 For example, infection of burned mice with wild-type P. aeruginosa kills the infected mice, whereas mice infected with a T3SS mutant of P. aeruginosa survive.31 In addition, passive immunization against one component of the T3SS of P. aeruginosa, PcrV, protects mice with severe burn wounds from P. aeruginosa infections.32 Immunization also ensures the survival of challenged mice and decreases lung inflammation and injury.18, 33 Inhibition of flagellar motility has also been shown to affect P. aeruginosa infection of burn wounds.
Today, the ability to treat bacterial infections has decreased because of intensive and constant exposure of bacteria to antibiotics. A potential strategy to overcome this challenge is to identify new drugs that block bacterial virulence factors without affecting bacterial growth.34 The T3SS has no human homologs, reducing the risk for mechanism-based side effects, and several families of natural and synthetic inhibitors of the T3SS in different pathogens have been reported.35, 36 The salicylidene acylhydrazide INP0341 belongs to a class of pharmacological agents that inhibits T3SS and flagellum, and thus disarms bacteria without killing them. Inhibition of T3SS and flagellum has been shown for bacteria such as Yersinia pseudotuberculosis,34 Salmonella typhimurium,37 Shigella spp.,38 Chlamydia spp.,39, 40 E. coli O157:H741 Pseudomonas aeruginosa42 and the plant pathogen Erwinia amylovora.43 Several authors suggest that a low probability for development of resistance is one of the key advantages of using virulence systems as targets for novel anti-infectives.44, 45
So far, a couple of in vivo studies have been published suggesting that T3SS inhibitors can lower the clinical symptoms caused by an infection,37, 46 and INP0341 has previously been shown to protect mice from vaginal Chlamydia trachomatis infection.
In this work, we show that the salicylidene acylhydrazide INP0341 inhibits the T3SS, the flagella motility system and the ability to form biofilm without affecting bacterial growth. In addition, INP0341 attenuates P. aeruginosa infection in a burn wound mouse model.
Materials and methods
Bacterial strains and growth conditions
Overnight cultures of the different bacterial strains (Supplementary Table 1) were grown in LB medium at 37 °C on a rotary shaker, if not otherwise stated. The OD of the bacterial cultures was measured on a Beckman Coulter DU530 spectrometer (Beckman Coulter AB, Sweden) at 595 nm. HeLa cells, DSMZ no. ACC 57, were kept in Dulbecco’s modified Eagle’s medium (GIBCO 61965) supplemented with 3 μg ml−1 of gentamicin and 10% fetal calf serum (VWR Sweden AB). The cells were subcultured three times a week.
Synthesis of INP0341
The virulence blocker INP034147 was synthesized as described previously48 and analytical data were in agreement with those previously reported.49 Stock solutions of INP0341 (20 mM) were prepared in DMSO, stored under dark and dry conditions and used for maximum 1 month before being discarded.
Bacterial growth assay
Overnight cultures of PAO1, PAK, PAKfliA::Gm and PAKexsA were diluted in LB (Luria Broth) to an OD595 of 0.1. The strains were seeded out in microtiter plates and 100, 50, 20 and 10 μM of INP0341 were added in triplicate to the different strains. OD595 were measured every hour for 6 h in a microplate reader (Tecan GENios, Tecan Trading AG, Switzerland). Wells with 1% DMSO without substance were used as positive controls.
Swimming assay
Overnight cultures of PAK and PAK fliA::Gm were diluted 10 times in LB and incubated for 3 h at 37 °C on a rotary shaker. Different concentrations of INP0341, dissolved in DMSO, were added to 0.3% liquid LB agar, 50 °C. The DMSO concentration was kept constant at 0.5% (v/v). The 0.3% liquid LB agar supplemented with 0.5% DMSO only was used as control. The warm LB agar solutions were added to the wells in 6-well plates and allowed to solidify for 4 h. A volume of 1 μl of bacterial culture, PAK or PAKfliA::Gm with an OD595 of 0.3 was added to the center of each well filled with 0.3% LB agar. The swimming zones were measured after overnight incubation at 30 °C.
Swarming assay
P. aeruginosa strain PAO1 was tested for swarming ability in the presence of INP0341 on 0.5% LB agar plates based on M8 medium supplemented with 0.2% glucose, 2 mM MgSO4 and 0.05% sodium glutamate as a nitrogen source. INP0341 was added at a final concentration of 5 μM in DMSO to the agar solution before the 6-well plates were solidified for 2 h. Overnight cultures of PAO1 were diluted to an OD595 of 0.3 in LB and 1 μl of the diluted bacteria were inoculated to the middle of each plate. The plates were incubated at 37 °C for 20 h. As controls, agar plates with 0.5% DMSO were used.
Biofilm assay
Static biofilms were grown at 37 °C for 24 h in a 96-well microtiter plate. Wells were inoculated with 100 μl of the overnight cultures of P. aeruginosa strains (wild-type and PAKfliA:Gm) diluted in 10% LB-phosphate-buffered saline (PBS) to an OD595 of 0.1. INP0341 was added to the wells at a final concentration of 10, 20, 50 or 100 μM. DMSO without substance was added to the controls. The plate was placed on a rotary shaker, 300 r.p.m., for 5 min for even distribution of the added substance. Samples from each experiment were carried out in triplicate, and repeated at least three times. The bacteria in the biofilm were visualized by washing the nonadherent bacteria with water and then staining the bound bacteria with 120 μl 0.1% crystal violet for 20 min followed by removal of unbound dye by immersing the plate in water three times. The stained biofilms were allowed to air-dry overnight. Addition of 150 μl ethanol was used to elute the dye and the optical density of each well was measured by a microtiter reader (Tecan GENios) at a wavelength of 595 nm.
Cytotoxicity assay
HeLa cells (2 × 105 cells per well) were seeded in a 96-well microtiter plate in Dulbecco’s modified Eagle’s medium containing 3 μg ml−1 gentamicin and 10% heat-inactivated fetal calf serum and incubated overnight at 37 °C, 5% CO2. Overnight cultures of the bacterial strains (PAK and PAKexsA) were grown in LB on a rotary shaker, 250 r.p.m. at 37 °C. Before infection, the HeLa cells were washed in PBS and 50 μl fetal calf serum free Dulbecco’s modified Eagle’s medium without phenol red and gentamicin but supplemented with the virulence blocker INP0341 at concentrations of 10, 20, 50 and 100 μM was added to the wells. Bacteria were diluted 1:10 and induced in Dulbecco’s modified Eagle’s medium at 37 °C for 1 h. After the induction, OD595 was measured. The infection was initiated by adding 50 μl of the bacteria to the HeLa cells diluted to a final concentration of OD595=0.002. The morphology of the cells was investigated with an inverted phase contrast microscopy at different time points following the infection. The T3S mutant PAKexsA was used as a negative control.
ExoS expression and secretion assay
Overnight cultures of the P. aeruginosa strain PAK was grown in brain heart infusion broth on a rotary shaker at 37 °C. The cultures were diluted 1:100 in fresh medium supplemented with 5 mM EGTA and 20 mM MgCl2. The virulence blocker INP0341 was added to the samples at concentrations of 50, 100 and 150 μM before incubation on a rotary shaker for 3 h at 37 °C. To compensate for the sample difference in growth rate during incubation, the culture OD595 was measured to allow calculation of individual loading volumes. Dithiothreitol was added to each of the collected samples that were placed on a 70 °C heating block for 10 min. The samples were then loaded on a NuPAGE 10% bis-Tris gel (Thermo Fisher Scientific, Sweden) depending on the OD595. The gel was blotted onto nitrocellulose membranes (Merck Chemicals GmbH, Germany) and blocked for 1 h in 1 × Tris-buffered saline with Tween-20 with 5% fat-free milk before incubation with primary α-ExoS antibodies (diluted 1:1000). ECL peroxidase-labeled secondary anti-rabbit antibodies (GE Healthcare, IL USA) were used at a concentration of 1:20 000. The protein bands were detected with the ECL plus western Blot detection system (GE Healthcare) and the chemiluminescence was recorded with an ECL camera (Nikon image station 2000r, Bergman Labora AB, Sweden). The band visualized in the ECL camera was compared with the expression and secretion levels from the wild-type control grown in the presence of DMSO but without addition of substance.
In vivo experiments
The 6-week-old female BalB/c mice were pretreated with cyclophosphamide and after 3 days a burn wound was initiated on the skin essentially as described previously.50 After the establishment of a burn wound the mice were pretreated with 500 μM INP0341 in 50% DMSO in PBS (5 mice) or 50% DMSO in PBS only (5 mice) onto the burn wound followed by infection with P. aeruginosa strain 180 (ATCC-19660) by adding 1 × 106 bacteria to the wound together with 70 μM INP0341 (NaOH 285 mM in PBS). In the first 24 h of infection, the mice were treated with 70 μM INP0341 (285 mM in PBS) four times and thereafter once every 12 h. Unlike human burn wounds, the burn wounds of mice are dry and heal fast, and therefore the wounds were left open. The cages were bedded with sawdust and special paper boxes and the mice had constant access to food and water. The mice were examined every 6 h and killed when showing life-threatening symptoms. Animal experiments were performed in an accredited establishment according to the governmental guidelines. Animal experiments were approved by the local Animal Experimentation Ethical Committee.
Results
INP0341 inhibits ExoS expression and secretion
As a first step, we investigated the effect of INP0341 on the expression and secretion levels of the virulence-associated protein ExoS. P. aeruginosa were cultivated with addition of INP0341 under T3SS-inducing conditions (Figure 1). The expression and secretion of ExoS by P. aeruginosa is induced in calcium-depleted media at 37 °C.51 Samples from both whole bacterial cultures (expressed) and supernatants (secreted) were analyzed by western blot using anti-ExoS antibodies. The samples from bacteria cultivated in calcium-depleted media in the absence of INP0341 showed protein levels that were significantly higher than samples cultivated in the presence of calcium. This confirms that calcium-depleted media induce the T3SS. Supernatants from bacteria cultured with INP0341 showed a dose-dependent decrease of the amount secreted ExoS compared with the DMSO control.
INP0341 inhibits ExoS expression and secretion. Expression and secretion of the T3S-toxin ExoS was induced in wild-type P. aeruginosa by depleting calcium from the growth medium. ExoS levels were measured in whole sample (expressed) and supernatant sample (secreted) by western blot using anti-ExoS antibodies (a). The structure of INP0341 (b).
INP0341 does not inhibit bacterial growth
To ensure that T3SS inhibition of INP0341 was not the result of general toxicity, the different P. aeruginosa strains were cultivated in the presence of the substance. Overnight cultures in LB were diluted and grown in a 96-well microplate in the presence of 100 μM of INP0341. The bacterial growth was measured every hour for 6 h. Wells with 1% DMSO without substance were used as controls. INP0341 did not inhibit growth of any of the tested P. aeruginosa strains (Supplementary Figure 1).
INP0341 inhibits the cytotoxic effect of P. aeruginosa on eukaryotic cells
The wild-type strain of P. aeruginosa (PAK) is known to cause a cytotoxic effect in the eukaryotic cell upon bacterial cell contact.51 This response is caused by delivery of effector molecules, exoenzymes, that are translocated into the host cells where they elicit different effects.25, 52 The bifunctional ExoS is best characterized and it has been shown that the enzyme affects the eukaryotic cells at many levels.25, 53, 54 To examine the protective effect of the virulence blocker, HeLa cells were infected with P. aeruginosa in the presence of INP0341 and the cytotoxic effect was studied after 3 and 7 h. Results from the wild-type DMSO control showed a changed in the morphology of the eukaryotic cell early upon bacterial infection. A complete rounding of the HeLa cells was observed after ∼7 h. Addition of INP0341 to HeLa cells before infection with the wild-type stain inhibited the rounding of the HeLa cells, a protective effect that was dose dependent (Figure 2). No toxic effect could be seen on the eukaryotic cells treated with INP0341 alone (data not shown). Both uninfected cells and cells infected with the PAKexsA mutant, a strain that lacks the ability to express any of the T3SS genes, were used as negative controls.1 None of these cells showed any tendency to round up at any time point during the experiment (Figure 2).
INP0341 inhibits the cytotoxic effect of P. aeruginosa on the eukaryotic cells. Epithelial cells were infected with wild-type P. aeruginosa and the protective effect of virulence blocker INP0341 was studied after 3 and 7 h. During infection the actin microfilaments were degraded by the exoenzymes and the cells showed a rounded morphology.
Inhibition of biofilm formation
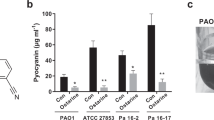
The wild-type and motility mutant strain PAKfliA::Gm55 were grown as biofilm in a microtiter plate under static conditions with 10% LB diluted in PBS as nutritional source. Environmental conditions (e.g., nutritional content of the medium) have been shown to play an important role in the formation of biofilm in vitro.56 To investigate the effect of INP0341 on biofilm formation, P. aeruginosa was incubated in the presence or absence of the INP0341 at concentrations of 0, 10, 20, 50 and 100 μM in a 96-well plate. DMSO was added to the controls. Cells organized in biofilms can be seen as a thin layer at the bottom of the wells or as a ring at the air liquid interface when stained with crystal violet. Ethanol was used to elute the dye from the formed biofilm and the OD of each well was measured by a microplate reader at a wavelength of 595 nm. The ability of P. aeruginosa to form biofilm in the presence of INP0341 was compared with bacteria incubated with DMSO only. The samples that contained INP0341 showed a dose-dependent inhibition of biofilm formation compared with the control samples according to Student’s t-test, P<0.05 (Figure 3). The mutant strain PAK was used as a negative control as it is deficient in the initial step in biofilm formation.15
Inhibition of P. aeruginosa biofilm by INP0341. Wild-type P. aeruginosa or a flagellar mutant (fliA::Gm) were grown for 24 h in 96-well plates with or without INP0341 and the biofilm stained with crystal violet and the relative absorbance was compared. Each bar represents mean values of three independent experiments in triplicate and the formation of biofilm by the wild-type P. aeruginosa was set to 100%. Addition of 50 and 100 μM INP0341 has a statistically significant inhibition on biofilm formation according to Student’s t-test, P<0.05.
Inhibition of swimming
Swimming is a flagella-dependent motility method used by several species of bacteria to move in liquids and soft agar plates. Flagella-dependent motility has shown to be important for the virulence of P. aeruginosa in animal models.57, 58 The flagella show homology with T3SS59 and these similarities between the two virulence systems and the importance of the flagella for bacterial virulence prompted us to investigate the effect of INP0341 on bacterial motility in a P. aeruginosa swimming assay. INP0341 reduced the swimming zone of P. aeruginosa, wild-type strain PAK already at 10 μM compared with the DMSO control (Figure 4). A PAKfliA::Gm, flagella mutant, was used as a negative control and could only move slightly in the agar.
Inhibition of P. aeruginosa swimming ability in the presence of INP0341. Bacteria were added to soft agar plates containing INP0341 at different concentrations and the swimming zones created by the bacteria were measured after 17 h. A reduction of swimming could be seen at 10 μM of INP0341. Values presented in the graphs are mean values from three separate experiments. Addition of 20 μM INP0341 has a statistically significant inhibition on biofilm formation according to Student’s t-test, P<0.01, and for 10 μM, P=0.05.
Inhibition of swarming
Swarming is used by P. aeruginosa and other Gram-negative bacteria to move across semisolid surfaces. Bacteria that swarm are hyper-flagellated and move in a coordinated manner, most likely in response to nutrients or by sensing the environment.60 P. aeruginosa requires the flagella and the type IV pili to be able to swarm, and this is not the case for other Gram-negative bacteria that use this type of movement.16 To investigate the effect on surface motility the swarming of the P. aeruginosa strain PAO1 was studied in the presence of INP0341. INP0341 inhibits swarming completely already at 5 μM compared with the DMSO control (Figure 5). An unflagellated mutant showed no swarming (data not shown).
In vivo efficacy
The effect of INP0341 on survival of mice infected P. aeruginosa was studied in a burn wound model essentially as described previously50 with BalB/c mice pretreated with cyclophosphamide. The wound was infected topically with P. aeruginosa. A solution of INP0341 was administered topically to the wound and the infection was followed for 72 h and the difference in survival between treated and untreated mice was investigated. We found that survival was prolonged for mice treated with INP0341 compared with untreated mice (Figure 6). The result is significant (P<0.05 according to log-rank Mantel–Cox test).
The survival of P. aeruginosa-infected burn wound mice pretreated and topically treated with INP0341. The mice were burn wounded and pretreated with 500 μM INP0341 in 50% DMSO. Thereafter, the mice were infected with P. aeruginosa strain 180 (1 × 106) and added to the wound together with 70 μM INP0341 in NaOH. The mice were treated with 70 μM INP0341 in NaOH every 12 h (except the first 24 h when the mice were treated 4 times) (5 mice/group). P<0.05 according to log-rank (Mantel–Cox) test.
Discussion
Antibiotic resistance is a large, emerging, worldwide problem and the need for new antimicrobial drugs is urgent. This challenge can be addressed by development of therapeutic agents based on virulence blocking compounds that disarm pathogenic bacteria without affecting bacterial growth. Many virulence systems are not found in endogenous bacteria and hence the normal flora will be left unaffected and resistance is less likely to evolve and spread in the normal flora. In addition, if the target for the virulence factor is located extracellular, the resistance caused by the highly active efflux pump system in P. aeruginosa will not be able to participate in the resistance development.
The reduced secretion of ExoS, one of the effector proteins present in the T3SS, by INP0341 shows that the substances affect an important virulence mechanism in P. aeruginosa. The inhibition of motility by INP0341 shows that it also interacts with the flagella system in P. aeruginosa. The T3SS is important in the initial phase of infection,28 whereas the flagella system is needed for the initial step of biofilm formation in P. aeruginosa.15, 61 Our in vitro results show that biofilm formation is reduced by adding INP0341 to the growth medium; consequently, the development of antibiotic resistance will be reduced as biofilm formation increases resistance 1000 times. Furthermore, we show that eukaryotic cells can be protected from a P. aeruginosa infection by addition of INP0341 to the growth medium without any toxic effect on eukaryotic cells. Finally, we could show that INP0341 can work as topical treatment for burn wound mice infected with P. aeruginosa. The topical treatment delayed the killing of the mice but INP0341 was not able to stop the systemic spread of bacteria. However, it might be possible to use the substance as preventive care in uninfected burn wounds. Because of solubility problems we were not able to increase the concentration of INP0341. It would have been interesting to see whether we could observe a better effect by increasing the dose. One problem when treating the animals topically is that the burn wounds of the mice become dry and hard very fast that might make it difficult for the substance to penetrate the wounds after a few hours. Therefore, we chose to treat the mice more often the first 24 h.
INP0341 belongs to the substance class salicylidene acylhydrazides. The pharmacokinetic properties of salicylidene acylhydrazides have previously been investigated.62 The small Cmax and short half-life makes it difficult to administrate sufficient amount of these substances systemically. Topical administration, as we have used in this study, is a strategy to overcome this problem and Chlamydia vaginal infection in mice has successfully been treated topically with salicylidene acylhydrazide.49, 63, 64 A formulation for topical administration that allows for slow release of INP0341 has been developed.63
The salicylidende acylhydrazones were found in a phenotypic screen34 and Wang et al.65 identified putative target proteins in E. coli by using an affinity reagent containing the salicylidene acylhydrazide attached to plastic beads. Of the 16 putative targets identified, the 3 most interesting proteins were deleted and the transcriptomes were investigated of each deletion strain. The transcriptomes showed that the flagella genes were upregulated and the T3SS-associated genes were downregulated. This suggests an indirect regulation of T3SS through upregulation of the flagella genes and indicates that INP0341 targets multiple proteins.65 Multiple targets increase the difficulty for the bacteria to develop resistance and another advantage is that the substances can act on multiple organisms. A drawback with multiple targets can be toxicity, but no toxicity could be seen on HeLa cells with the addition of INP0341.
Slepenkin et al.47 studied the mechanism of INP0341 on Chlamydia and found that the substance has an effect on the T3SS gene expression during middle to late events in the Chlamydial development cycle. Salicylidene acylhydrazides can chelate metal ions and it is found that that the anti-chlamydial effect of INP0341 is the result of depletion of intracellular iron. Many pathogenic bacteria are sensitive to iron starvation and removal Fe(III) is a method used by the human body to control infections.66 Chu et al.64 have shown that INP0341 can function as a vaginal microbicide as it inhibits both C. trachomatis and N. gonorrhoeae without effecting the normal vaginal flora. Furthermore, INP0341 has anti HIV-activity in vitro67 and consequently INP0341 and other salicylidende acylhydrazides are interesting candidates for development of general vaginal microbicides. A study by Rzhepishevska et al.68 showed that the inhibitory effect of the Fe(III) antagonist Ga(III) on biofilm formation was increased when a salicylidene acylhydrazide (ME0163) was in a complex with Ga(III) and at the same time suppressing the T3SS in P. aeruginosa. ME0163 has only a minor effect on the T3SS by itself and a recent study with INP0341 together with Ga(III) showed a different pattern on antibiofilm activity and ExoS secretion compared with the ME0163 complex.69 The difference between the two hydrazine-Ga(III) complexes with similar affinity for Ga(III) argues for additional target and not only iron-chelating properties, although Gallium maltolate, a gallium formulation, has shown antibacterial activity against P. aeruginosa in thermally injured mice.70
In conclusion, we can disarm P. aeruginosa by inhibiting the T3SS and the flagella system with the virulence blocking agent INP0341. Finally, we also inhibit biofilm formation and attenuate P. aeruginosa burn wound infection in mice by a topical treatment with INP0341. Our findings show that the salicylidende acylhydrazides are capable of attenuating virulence in the highly pathogenic-resistant pathogen P. aeruginosa.
References
Frank, D. W. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol. Microbiol. 26, 621–629 (1997).
Lyczak, J. B., Cannon, C. L. & Pier, G. B. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2, 1051–1060 (2000).
Hauser, A. R. & Sriram, P. Severe Pseudomonas aeruginosa infections. Tackling the conundrum of drug resistance. Postgrad. Med. 117, 41–48 (2005).
Govan, J. R. & Nelson, J. W. Microbiology of lung infection in cystic fibrosis. Br. Med. Bull. 48, 912–930 (1992).
Bodey, G. P., Bolivar, R., Fainstein, V. & Jadeja, L. Infections caused by Pseudomonas aeruginosa. Rev. Infect. Dis. 5, 279–313 (1983).
Sundin, C., Henriksson, M. L., Hallberg, B., Forsberg, A. & Frithz-Lindsten, E. Exoenzyme T of Pseudomonas aeruginosa elicits cytotoxicity without interfering with Ras signal transduction. Cell. Microbiol. 3, 237–246 (2001).
Rocchetta, H. L., Burrows, L. L. & Lam, J. S. Genetics of O-antigen biosynthesis in Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 63, 523–553 (1999).
Lambert, P. A. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa. J. R. Soc. Med. 95, 22–26 (2002).
Hoyle, B. D. & Costerton, J. W. Bacterial resistance to antibiotics: the role of biofilms. Prog. Drug Res. 37, 91–105 (1991).
Altoparlak, U., Erol, S., Akcay, M. N., Celebi, F. & Kadanali, A. The time-related changes of antimicrobial resistance patterns and predominant bacterial profiles of burn wounds and body flora of burned patients. Burns 30, 660–664 (2004).
Stoodley, P., Cargo, R., Rupp, C. J., Wilson, S. & Klapper, I. Biofilm material properties as related to shear-induced deformation and detachment phenomena. J. Ind. Microbiol. Biotechnol. 29, 361–367 (2002).
Sutherland, I. W. The biofilm matrix—an immobilized but dynamic microbial environment. Trends Microbiol. 9, 222–227 (2001).
Watnick, P. & Kolter, R. Biofilm, city of microbes. J. Bacteriol. 182, 2675–2679 (2000).
Armitage, G. C. Basic features of biofilms—why are they difficult therapeutic targets? Ann. R. Australas Coll. Dent. Surg. 17, 30–34 (2004).
O'Toole, G. A. & Kolter, R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30, 295–304 (1998).
Kohler, T., Curty, L. K., Barja, F., van Delden, C. & Pechere, J. C. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol. 182, 5990–5996 (2000).
Semmler, A. B., Whitchurch, C. B. & Mattick, J. S. A re-examination of twitching motility in Pseudomonas aeruginosa. Microbiology 145 (Pt 10), 2863–2873 (1999).
Sawa, T. et al. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat. Med. 5, 392–398 (1999).
Dacheux, D., Attree, I., Schneider, C. & Toussaint, B. Cell death of human polymorphonuclear neutrophils induced by a Pseudomonas aeruginosa cystic fibrosis isolate requires a functional type III secretion system. Infect. Immun. 67, 6164–6167 (1999).
Hueck, C. J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62, 379–433 (1998).
Schesser, K., Francis, M. S., Forsberg, A., Wolf-Watz, H. in Cellular Microbiology (eds Cossart, P., Boguet, P., Normark, S. & Rappuoli, P. 239–263 American Society for Microbiology Press, Washington, (2000).
Rosqvist, R., Magnusson, K. E. & Wolf-Watz, H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13, 964–972 (1994).
Keyser, P., Elofsson, M., Rosell, S. & Wolf-Watz, H. Virulence blockers as alternatives to antibiotics: type III secretion inhibitors against Gram-negative bacteria. J. Intern. Med. 264, 17–29 (2008).
Pederson, K. J. & Barbieri, J. T. Intracellular expression of the ADP-ribosyltransferase domain of Pseudomonas exoenzyme S is cytotoxic to eukaryotic cells. Mol. Microbiol. 30, 751–759 (1998).
Frithz-Lindsten, E., Du, Y., Rosqvist, R. & Forsberg, A. Intracellular targeting of exoenzyme S of Pseudomonas aeruginosa via type III-dependent translocation induces phagocytosis resistance, cytotoxicity and disruption of actin microfilaments. Mol. Microbiol. 25, 1125–1139 (1997).
Hornef, M. W. et al. Triggering the ExoS regulon of Pseudomonas aeruginosa: a GFP-reporter analysis of exoenzyme (Exo) S, ExoT and ExoU synthesis. Microb. Pathog. 29, 329–343 (2000).
Sundin, C., Hallberg, B. & Forsberg, A. ADP-ribosylation by exoenzyme T of Pseudomonas aeruginosa induces an irreversible effect on the host cell cytoskeleton in vivo. FEMS Microbiol. Lett. 234, 87–91 (2004).
Hogardt, M., Roeder, M., Schreff, A. M., Eberl, L. & Heesemann, J. Expression of Pseudomonas aeruginosa exoS is controlled by quorum sensing and RpoS. Microbiology 150, 843–851 (2004).
Arora, S. K., Neely, A. N., Blair, B., Lory, S. & Ramphal, R. Role of motility and flagellin glycosylation in the pathogenesis of Pseudomonas aeruginosa burn wound infections. Infect. Immun. 73, 4395–4398 (2005).
Roy-Burman, A. et al. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J. Infect. Dis. 183, 1767–1774 (2001).
Holder, I. A., Neely, A. N. & Frank, D. W. Type III secretion/intoxication system important in virulence of Pseudomonas aeruginosa infections in burns. Burns 27, 129–130 (2001).
Neely, A. N., Holder, I. A., Wiener-Kronish, J. P. & Sawa, T. Passive anti-PcrV treatment protects burned mice against Pseudomonas aeruginosa challenge. Burns 31, 153–158 (2005).
Imamura, Y. et al. Effect of anti-PcrV antibody in a murine chronic airway Pseudomonas aeruginosa infection model. Eur. Respir. J. 29, 965–968 (2007).
Kauppi, A. M., Nordfelth, R., Uvell, H., Wolf-Watz, H. & Elofsson, M. Targeting bacterial virulence: inhibitors of type III secretion in Yersinia. Chem. Biol. 10, 241–249 (2003).
Gu, L., Zhou, S., Zhu, L., Liang, C. & Chen, X. Small-Molecule Inhibitors of the Type III Secretion System. Molecules 20, 17659–17674 (2015).
Duncan, M. C., Linington, R. G. & Auerbuch, V. Chemical inhibitors of the type three secretion system: disarming bacterial pathogens. Antimicrob. Agents Chemother. 56, 5433–5441 (2012).
Hudson, D. L. et al. Inhibition of type III secretion in Salmonella enterica serovar Typhimurium by small-molecule inhibitors. Antimicrob. Agents Chemother. 51, 2631–2635 (2007).
Veenendaal, A. K., Sundin, C. & Blocker, A. J. Small-molecule type III secretion system inhibitors block assembly of the Shigella type III secreton. J. Bacteriol. 191, 563–570 (2009).
Muschiol, S. et al. A small-molecule inhibitor of type III secretion inhibits different stages of the infectious cycle of Chlamydia trachomatis. Proc. Natl Acad. Sci. USA 103, 14566–14571 (2006).
Bailey, L. et al. Small molecule inhibitors of type III secretion in Yersinia block the Chlamydia pneumoniae infection cycle. FEBS Lett. 581, 587–595 (2007).
Tree, J. J. et al. Characterization of the effects of salicylidene acylhydrazide compounds on type III secretion in Escherichia coli O157:H7. Infect. Immun. 77, 4209–4220 (2009).
Anantharajah, A. et al. Salicylidene acylhydrazides and hydroxyquinolines act as inhibitors of type three secretion systems in Pseudomonas aeruginosa by distinct mechanisms. Antimicrob. Agents Chemother. (e-pub ahead of print 10 April 2017; doi:10.1128/AAC.02566-16.
Yang, F. et al. Small-molecule inhibitors suppress the expression of both type III secretion and amylovoran biosynthesis genes in Erwinia amylovora. Mol. Plant Pathol. 15, 44–57 (2014).
Clatworthy, A. E., Pierson, E. & Hung, D. T. Targeting virulence: a new paradigm for antimicrobial therapy. Nat. Chem. Biol. 3, 541–548 (2007).
Marra, A. Targeting virulence for antibacterial chemotherapy: identifying and characterising virulence factors for lead discovery. Drugs R D 7, 1–16 (2006).
Kimura, K. et al. A small-molecule inhibitor of the bacterial type III secretion system protects against in vivo infection with Citrobacter rodentium. J. Antibiot. (Tokyo) 64, 197–203 (2011).
Slepenkin, A. et al. Reversal of the antichlamydial activity of putative type III secretion inhibitors by iron. Infect. Immun. 75, 3478–3489 (2007).
Dahlgren, M. K., Kauppi, A. M., Olsson, I. M., Linusson, A. & Elofsson, M. Design, synthesis, and multivariate quantitative structure-activity relationship of salicylanilides—potent inhibitors of type III secretion in Yersinia. J. Med. Chem. 50, 6177–6188 (2007).
Slepenkin, A., Chu, H., Elofsson, M., Keyser, P. & Peterson, E. M. Protection of mice from a Chlamydia trachomatis vaginal infection using a Salicylidene acylhydrazide, a potential microbicide. J. Infect. Dis. 204, 1313–1320 (2011).
Shen, Y. et al. Plasminogen is a key proinflammatory regulator that accelerates the healing of acute and diabetic wounds. Blood 119, 5879–5887 (2012).
Sundin, C., Wolfgang, M. C., Lory, S., Forsberg, A. & Frithz-Lindsten, E. Type IV pili are not specifically required for contact dependent translocation of exoenzymes by Pseudomonas aeruginosa. Microb. Pathog. 33, 265–277 (2002).
Garrity-Ryan, L. et al. The arginine finger domain of ExoT contributes to actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect. Immun. 68, 7100–7113 (2000).
Ganesan, A. K., Frank, D. W., Misra, R. P., Schmidt, G. & Barbieri, J. T. Pseudomonas aeruginosa exoenzyme S ADP-ribosylates Ras at multiple sites. J. Biol. Chem. 273, 7332–7337 (1998).
Goehring, U. M., Schmidt, G., Pederson, K. J., Aktories, K. & Barbieri, J. T. The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J. Biol. Chem. 274, 36369–36372 (1999).
Starnbach, M. N. & Lory, S. The fliA (rpoF) gene of Pseudomonas aeruginosa encodes an alternative sigma factor required for flagellin synthesis. Mol. Microbiol. 6, 459–469 (1992).
Klausen, M. et al. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 48, 1511–1524 (2003).
Montie, T. C., Doyle-Huntzinger, D., Craven, R. C. & Holder, I. A. Loss of virulence associated with absence of flagellum in an isogenic mutant of Pseudomonas aeruginosa in the burned-mouse model. Infect. Immun. 38, 1296–1298 (1982).
Holder, I. A., Wheeler, R. & Montie, T. C. Flagellar preparations from Pseudomonas aeruginosa: animal protection studies. Infect. Immun. 35, 276–280 (1982).
Blocker, A., Komoriya, K. & Aizawa, S. Type III secretion systems and bacterial flagella: insights into their function from structural similarities. Proc. Natl Acad. Sci. USA 100, 3027–3030 (2003).
Harshey, R. M. Bees aren't the only ones: swarming in gram-negative bacteria. Mol. Microbiol. 13, 389–394 (1994).
Toutain, C. M., Caizza, N. C., Zegans, M. E. & O'Toole, G. A. Roles for flagellar stators in biofilm formation by Pseudomonas aeruginosa. Res. Microbiol. 158, 471–477 (2007).
Ur-Rehman, T. et al. Pre-clinical pharmacokinetics and anti-chlamydial activity of salicylidene acylhydrazide inhibitors of bacterial type III secretion. J. Antibiot. (Tokyo) 65, 397–404 (2012).
Pedersen, C. et al. Formulation of the microbicide INP0341 for in vivo protection against a vaginal challenge by Chlamydia trachomatis. PLoS ONE 9, e110918 (2014).
Chu, H. et al. Candidate vaginal microbicides with activity against Chlamydia trachomatis and Neisseriagonorrhoeae. Int. J. Antimicrob. Agents 36, 145–150 (2010).
Wang, D. et al. Identification of bacterial target proteins for the salicylidene acylhydrazide class of virulence-blocking compounds. J. Biol. Chem. 286, 29922–29931 (2011).
Bullen, J. J., Rogers, H. J., Spalding, P. B. & Ward, C. G. Iron and infection: the heart of the matter. FEMS Immunol. Med. Microbiol. 43, 325–330 (2005).
Forthal, D. N. et al. In vitro anti-HIV-1 activity of salicylidene acylhydrazide compounds. Int. J. Antimicrob. Agents 40, 354–360 (2012).
Rzhepishevska, O. et al. The gallium(III)–salicylidene acylhydrazide complex shows synergistic anti-biofilm effect and inhibits toxin production by Pseudomonas aeruginosa. J. Inorg. Biochem. 138, 1–8 (2014).
Hakobyan, S., Rzhepishevska, O., Bjorn, E., Boily, J. F. & Ramstedt, M. Influence of chelation strength and bacterial uptake of gallium salicylidene acylhydrazide on biofilm formation and virulence of Pseudomonas aeruginosa. J. Inorg. Biochem. 160, 24–32 (2016).
DeLeon, K. et al. Gallium maltolate treatment eradicates Pseudomonas aeruginosa infection in thermally injured mice. Antimicrob. Agents Chemother. 53, 1331–1337 (2009).
Acknowledgements
ME is grateful for support from the Swedish Research Council and Umeå Centre for Microbial Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Uusitalo, P., Hägglund, U., Rhöös, E. et al. The salicylidene acylhydrazide INP0341 attenuates Pseudomonas aeruginosa virulence in vitro and in vivo. J Antibiot 70, 937–943 (2017). https://doi.org/10.1038/ja.2017.64
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2017.64
This article is cited by
-
Pharmacokinetics, tissue distribution, bioavailability and excretion of the anti-virulence drug Fluorothiazinon in rats and rabbits
The Journal of Antibiotics (2024)
-
Using next generation antimicrobials to target the mechanisms of infection
npj Antimicrobials and Resistance (2023)
-
Exploring resveratrol dimers as virulence blocking agents – Attenuation of type III secretion in Yersinia pseudotuberculosis and Pseudomonas aeruginosa
Scientific Reports (2020)
-
Identification of the natural product paeonol derived from peony bark as an inhibitor of the Salmonella enterica serovar Typhimurium type III secretion system
Applied Microbiology and Biotechnology (2020)
-
Regulation and controlling the motility properties of Pseudomonas aeruginosa
Applied Microbiology and Biotechnology (2020)