Abstract
Background and aims:
Gastric vagal afferents convey satiety signals in response to mechanical stimuli. The sensitivity of these afferents is decreased in diet-induced obesity. Leptin, secreted from gastric epithelial cells, potentiates the response of vagal afferents to mechanical stimuli in lean mice, but has an inhibitory effect in high-fat diet (HFD)-induced obese mice. We sought to determine whether changes in vagal afferent function and response to leptin in obesity were reversible by returning obese mice consuming a HFD to standard laboratory chow diet (SLD).
Methods:
Eight-week-old female C57BL/6 mice were either fed a SLD (N=20) or HFD (N=20) for 24 weeks. A third group was fed a HFD for 12 weeks and then a SLD for a further 12 weeks (RFD, N=18). An in vitro gastro-oesophageal vagal afferent preparation was used to determine the mechanosensitivity of gastric vagal afferents and the modulatory effect of leptin (0.1–10 nM) was examined. Retrograde tracing and quantitative RT–PCR were used to determine the expression of leptin receptor (LepR) messenger RNA (mRNA) in whole nodose and specific cell bodies traced from the stomach.
Results:
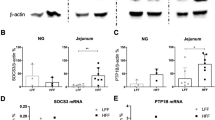
After 24 weeks, both the HFD and RFD mice had increased body weight, gonadal fat mass, plasma leptin, plasma insulin and daily energy consumption compared with the SLD mice. The HFD and RFD mice had reduced tension receptor mechanosensitivity and leptin further inhibited responses to tension in HFD, RFD but not SLD mice. Mucosal receptors from both the SLD and RFD mice were potentiated by leptin, an effect not seen in HFD mice. LepR expression was unchanged in the whole nodose, but was reduced in the mucosal afferents of the HFD and RFD mice.
Conclusion:
Disruption of gastric vagal afferent function by HFD-induced obesity is only partially reversible by dietary change, which provides a potential mechanism preventing maintenance of weight loss.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Wing RR, Phelan S . Long-term weight loss maintenance. Am J Clin Nutr 2005; 82: 222S–225S.
Wadden TA, Frey DL . A multicenter evaluation of a proprietary weight loss program for the treatment of marked obesity: a five-year follow-up. Int J Eat Disord 1997; 22: 203–212.
Skender ML, Goodrick GK, Del Junco DJ, Reeves RS, Darnell L, Gotto AM Jr et al. Comparison of 2-Year weight loss trends in behavioral treatments of obesity: diet, exercise, and combination interventions. J Am Diet Assoc 1996; 96: 342–346.
Tsai AG, Wadden TA . Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med 2005; 142: 56–66.
Kennedy GC . The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci 1953; 140: 578–592.
Woods SC, Porte D, Bobbioni E, Ionescu E, Sauter JF, Rohner-Jeanrenaud F et al. Insulin: its relationship to the central nervous system and to the control of food intake and body weight. Am J Clin Nutr 1985; 42: 1063–1071.
Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A et al. Long-term persistence of hormonal adaptations to weight loss. New Engl J Med 2011; 365: 1597–1604.
Stanley S, Wynne K, McGowan B, Bloom S . Hormonal regulation of food intake. Physiol Rev 2005; 85: 1131–1158.
Inui A, Asakawa A, Bowers CY, Mantovani G, Laviano A, Meguid MM et al. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J 2004; 18: 439–456.
Havel PJ . Peripheral signals conveying metabolic information to the brain: short-term and long-term regulation of food intake and energy homeostasis. Exp Biol Med 2001; 226: 963–977.
Meyer JH, Hlinka M, Tabrizi Y, DiMaso N, Raybould HE . Chemical specificities and intestinal distributions of nutrient-driven satiety. Am J Physiol 1998; 275: R1293–R1307.
D'Alessio D . Intestinal hormones and regulation of satiety: the case for CCK, GLP-1, PYY, and Apo A-IV. J Parenter Enteral Nutr 2008; 32: 567–568.
Blackshaw LA, Grundy D, Scratcherd T . Vagal afferent discharge from gastric mechanoreceptors during contraction and relaxation of the ferret corpus. J Auton Nerv Syst 1987; 18: 19–24.
Read NW . Role of gastrointestinal factors in hunger and satiety in man. Proc Nutr Soc 1992; 51: 7–11.
Page AJ, Martin CM, Blackshaw LA . Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol 2002; 87: 2095–2103.
Wang G, Tomasi D, Backus W, Wang R, Telang F, Geliebter A et al. Gastric distention activates satiety circuitry in the human brain. Neuroimage 2008; 39: 1824–1831.
Tanaka T, Kendrick ML, Zyromski NJ, Meile T, Sarr MG . Vagal innervation modulates motor pattern but not initiation of canine gastric migrating motor complex. Am J Physiology Gastrointest Liver Physiol 2001; 281: G283–G292.
Becker JM, Kelly KA . Antral control of canine gastric emptying of solids. Am J Physiol Gastrointest Liver Physiol 1983; 8: 334–338.
Kentish S, Li H, Philp LK, O’Donnell TA, Isaacs NJ, Young RL et al. Diet-induced adaptation of vagal afferent function. J Physiol 2012; 590: 209–221.
Daly DM, Park SJ, Valinsky WC, Beyak MJ . Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. J Physiol 2011; 589: 2857–2870.
Rentsch J, Levens N, Chiesi M . Recombinant OB-gene product reduces food-intake in fasted mice. Biochem Biophys Res Commun 1995; 214: 131–136.
Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau J, Bortoluzzi MI et al. The stomach is a source of leptin. Nature 1998; 394: 790–793.
Satoh N, Ogawa Y, Katsuura G, Hayase M, Tsuji T, Imagawa K et al. The arcuate nucleus as a primary site of satiety effect of leptin in rats. Neurosci Lett 1997; 224: 149–152.
Buyse M, Ovesjö M-L, Goïot H, Guilmeau S, Péranzi G, Moizo L et al. Expression and regulation of leptin receptor proteins in afferent and efferent neurons of the vagus nerve. Eur J Neurosci 2001; 14: 64–72.
Peters JH, McKay BM, Simasko SM, Ritter RC . Leptin-induced satiation mediated by abdominal vagal afferents. Am J Physiol Regul Integr Comp Physiol 2005; 288: 879–884.
de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE . Leptin resistance in vagal afferent neurons inhibits cholecystokinin signaling and satiation in diet induced obese rats. PloS One 2012; 7: e32967.
de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE . Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am J Physiol Endocrinol Metab 2011; 301: E187–E195.
Kentish SJ, O'Donnell TA, Isaacs NJ, Young RL, Li H, Harrington AM et al. Gastric vagal afferent modulation by leptin is influenced by food intake status. J Physiol 2012; 591 (Pt 7): 1921–1934.
Hughes PA, Brierley SM, Young RL, Blackshaw LA . Localization and comparative analysis of acid-sensing ion channel (ASIC1, 2, and 3) mRNA expression in mouse colonic sensory neurons within thoracolumbar dorsal root ganglia. J Comp Neurol 2007; 500: 863–875.
Pfaffl MW, Horgan GW, Dempfle L . Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 2002; 30: e36.
Tomasi D, Wang G-J, Wang R, Backus W, Geliebter A, Telang F et al. Association of body mass and brain activation during gastric distention: implications for obesity. PloS One 2009; 4: e6847.
Sainsbury A, Zhang L . Role of the hypothalamus in the neuroendocrine regulation of body weight and composition during energy deficit. Obes Rev 2012; 13: 234–257.
Lemonnier D, Suquet JP, Aubert R, De Gasquet P, Pequignot E . Metabolism of the mouse made obese by a high-fat diet. Diabete Metab 1975; 1: 77–85.
Ravussin Y, Gutman R, Diano S, Shanabrough M, Borok E, Sarman B et al. Effects of chronic weight perturbation on energy homeostasis and brain structure in mice. Am J Physiol Regul, Integr Comp Physiol 2011; 300: R1352–R1362.
Levin BE, Dunn-Meynell AA . Defense of body weight against chronic caloric restriction in obesity-prone and -resistant rats. Am J Physiol Regul, Integr Comp Physiol 2000; 278: R231–R237.
DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM et al. Persistence of abnormal neural responses to a meal in postobese individuals. Int J Obes Relat Metab Disord 2004; 28: 370–377.
Apolzan JW, Harris RBS . Rapid onset and reversal of peripheral and central leptin resistance in rats offered chow, sucrose solution, and lard. Appetite 2013; 60: 65–73.
Cammisotto PG, Bendayan M, Levy E . Regulation of leptin receptor expression in human polarized Caco-2/15 cells. Endocr Metab Immune Disord Drug Targets 2012; 12: 57–70.
Gatta B, Nunes ML, Bailacq-Auder C, Etchechoury L, Collet D, Tabarin A . Is bariatric surgery really inefficient in hypothalamic obesity? Clin Endocrinol 2013; 78: 636–638.
Inge TH, Pfluger P, Zeller M, Rose SR, Burget L, Sundararajan S et al. Gastric bypass surgery for treatment of hypothalamic obesity after craniopharyngioma therapy. Nature clinical practice. Endocrinol Metab 2007; 3: 606–609.
Nilsson C, Raun K, Yan FF, Larsen MO, Tang-Christensen M . Laboratory animals as surrogate models of human obesity. Acta pharmacologica Sinica 2012; 33: 173–181.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Author Contributions
SJK was responsible for performing electrophysiology experiments, ELISA assays and writing the manuscript. TAO’D monitored the weight and food intake of the mice. CLF performed the QRT-PCR experiments. HL performed the retrograde tracing. GAW provided intellectual input into the design, and interpretation of the experiments and assisted with the preparation of the manuscript. AJP collected and processed the tissue samples, co-designed experiments and aided in the preparation of the manuscript.
Rights and permissions
About this article
Cite this article
Kentish, S., O'Donnell, T., Frisby, C. et al. Altered gastric vagal mechanosensitivity in diet-induced obesity persists on return to normal chow and is accompanied by increased food intake. Int J Obes 38, 636–642 (2014). https://doi.org/10.1038/ijo.2013.138
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2013.138
Keywords
This article is cited by
-
Novel Insights into the Physiology of Nutrient Sensing and Gut-Brain Communication in Surgical and Experimental Obesity Therapy
Obesity Surgery (2023)
-
Neural signalling of gut mechanosensation in ingestive and digestive processes
Nature Reviews Neuroscience (2022)
-
The metabolic role of vagal afferent innervation
Nature Reviews Gastroenterology & Hepatology (2018)